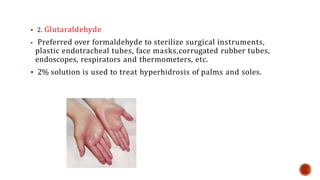
This document discusses antiseptics and disinfectants. It defines antiseptics as agents used on living tissues to eliminate microorganisms, while disinfectants are used on inanimate objects. Some common antiseptics and disinfectants mentioned include phenol derivatives, halogens, alcohols, aldehydes, and surface active agents. The document also outlines ideal properties of antiseptics/disinfectants and provides details on specific agents such as chlorhexidine, iodine, alcohol, formaldehyde, and hydrogen peroxide.