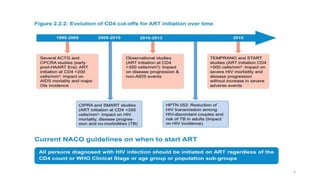
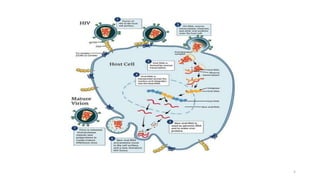
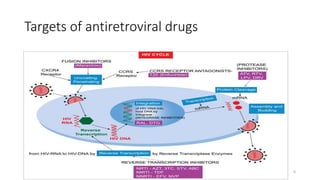
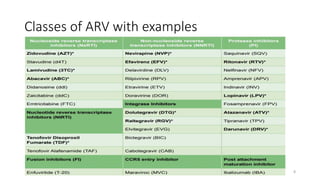
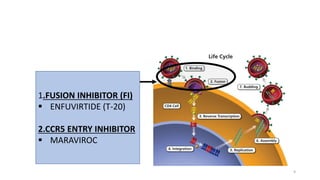
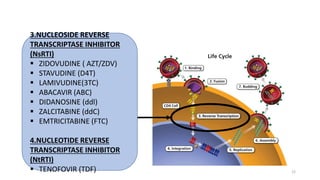
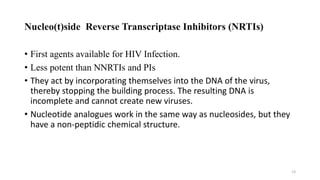
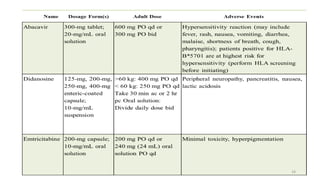
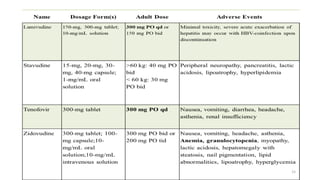
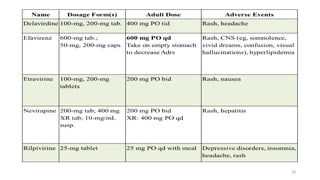
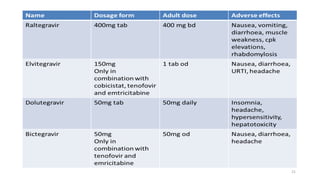
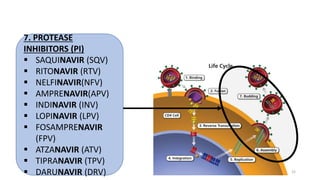
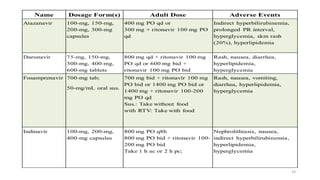
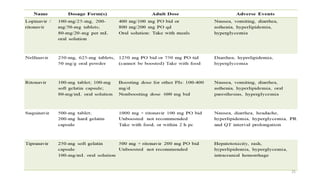
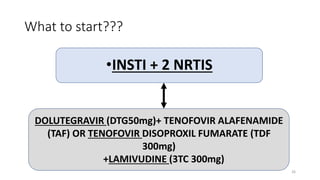
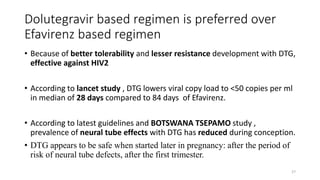
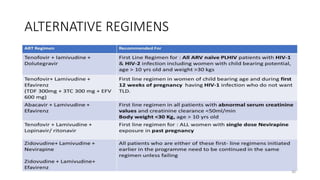
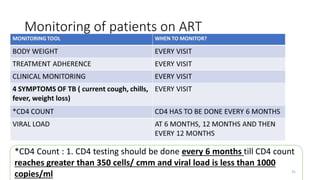
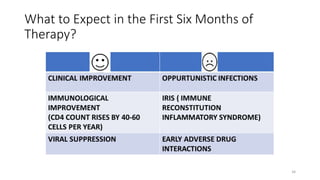
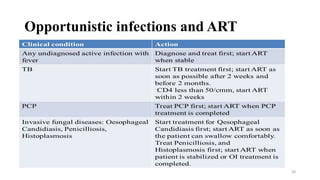
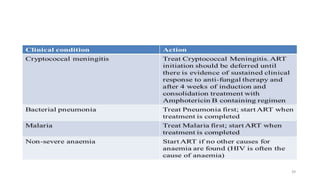
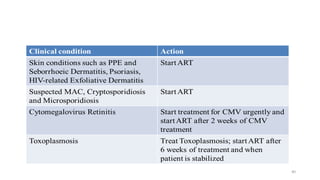
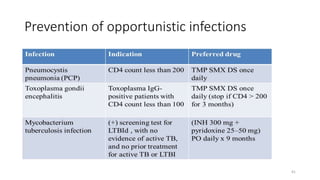
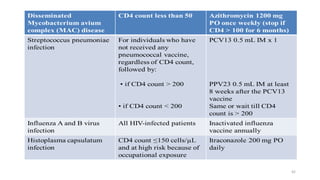
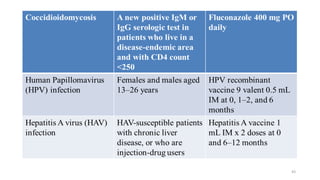
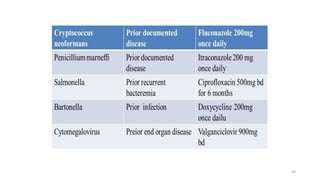
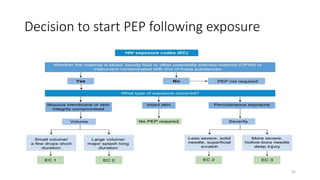
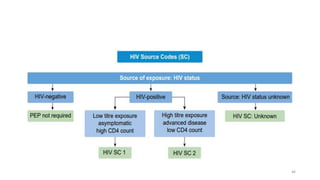
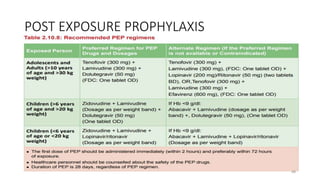
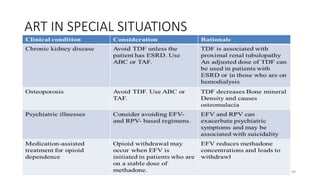
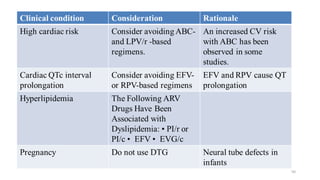
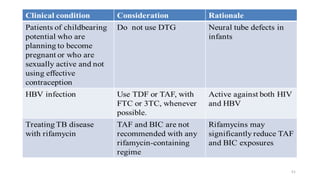
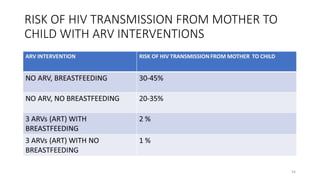
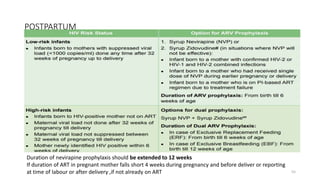
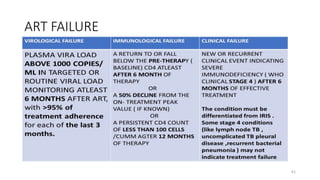
This document discusses antiretroviral therapy (ART) for HIV. It outlines the goals of ART as treating all patients regardless of clinical stage or CD4 count. It describes the different classes of antiretroviral drugs including entry inhibitors, nucleoside/nucleotide reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, integrase inhibitors, and protease inhibitors. It recommends the preferred first-line regimen of dolutegravir plus tenofovir/lamivudine and discusses monitoring patients on ART and managing opportunistic infections.