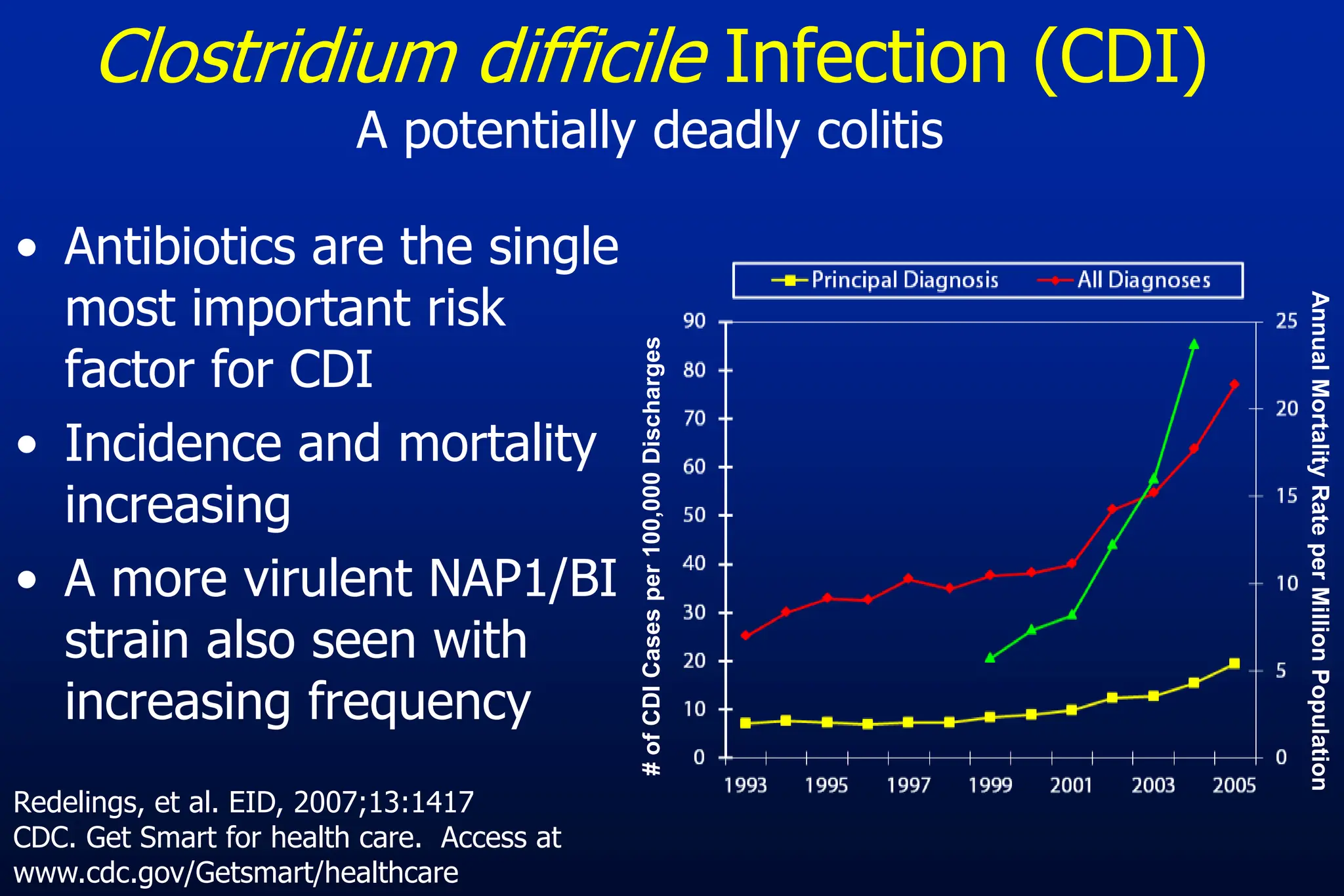
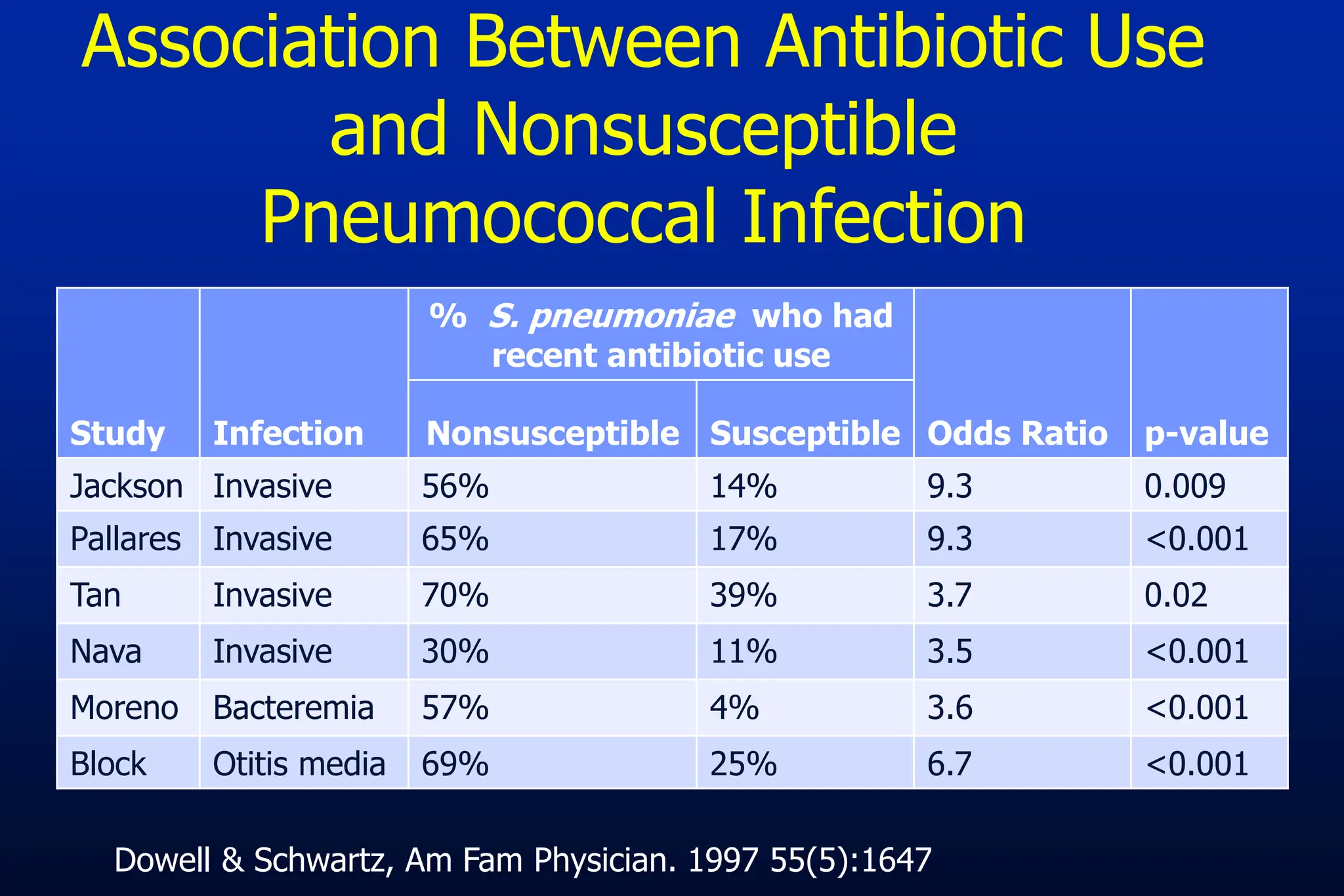
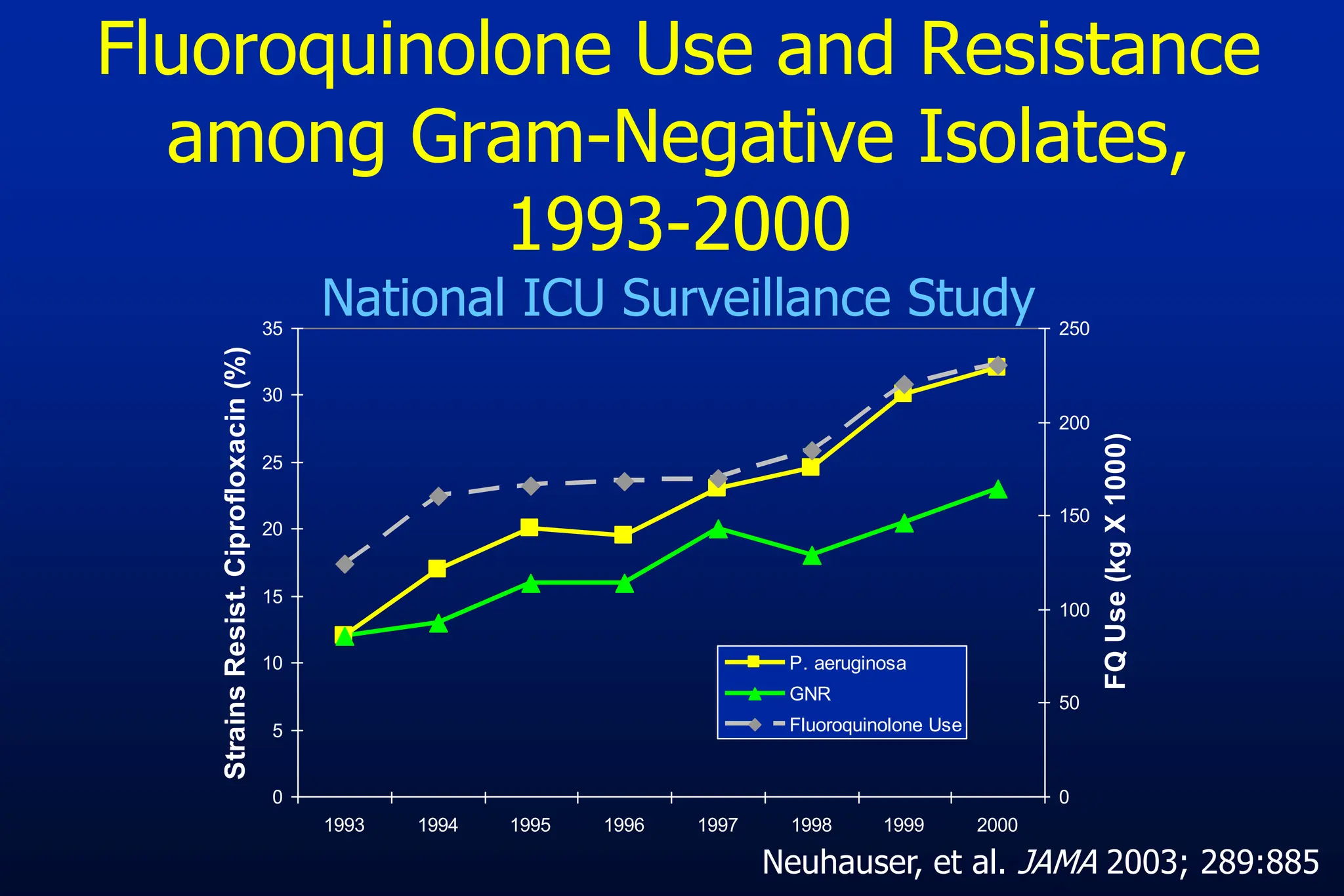
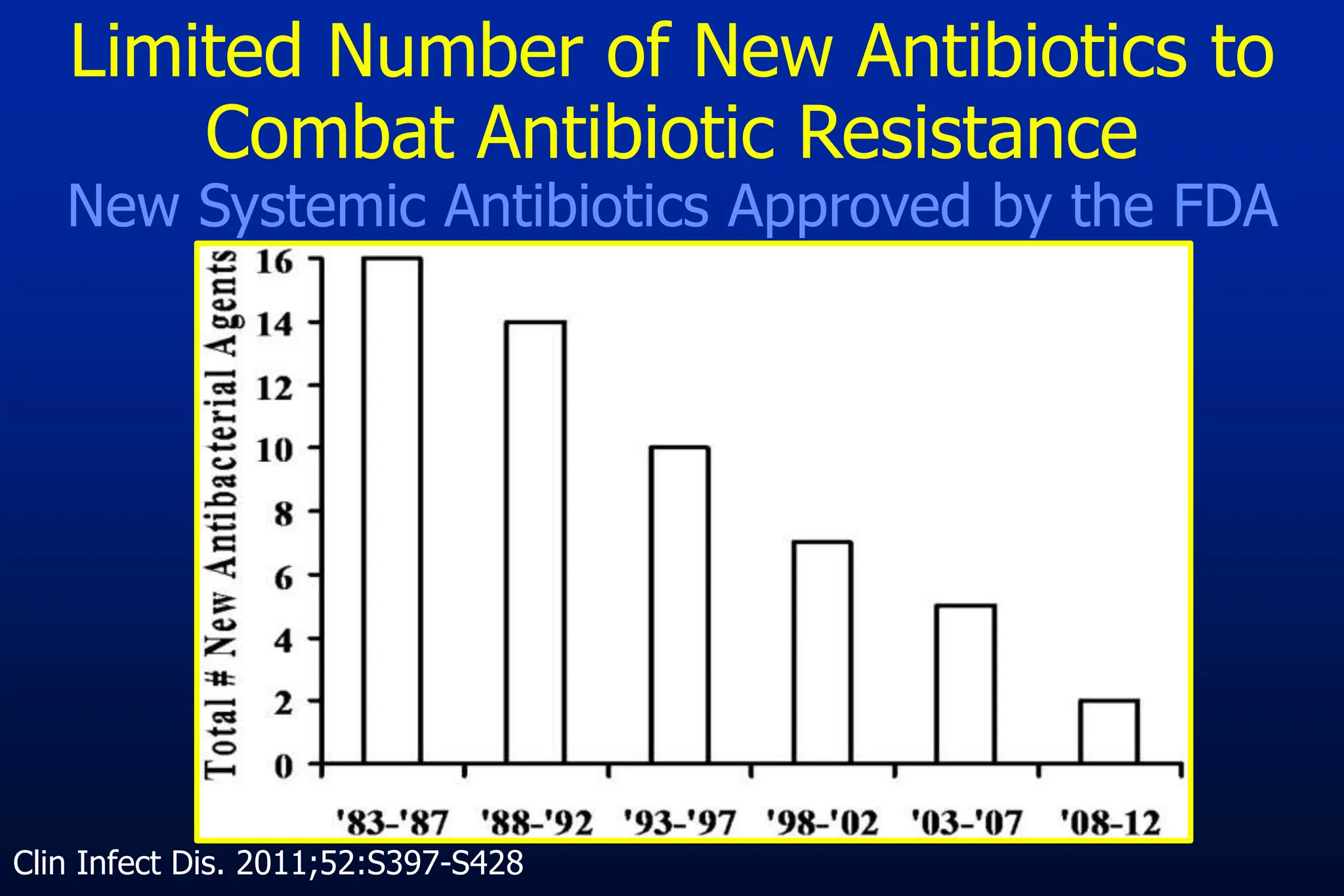
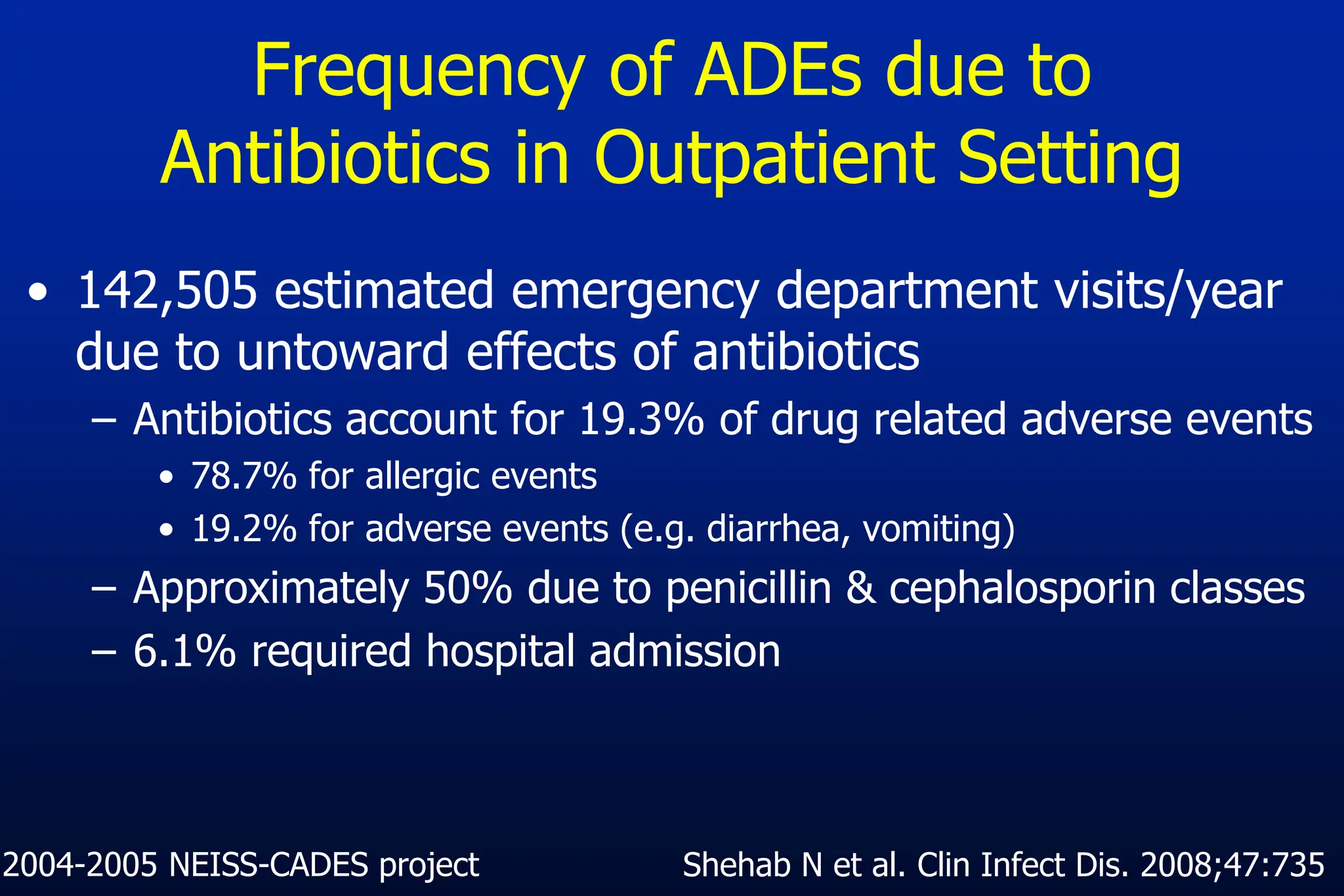
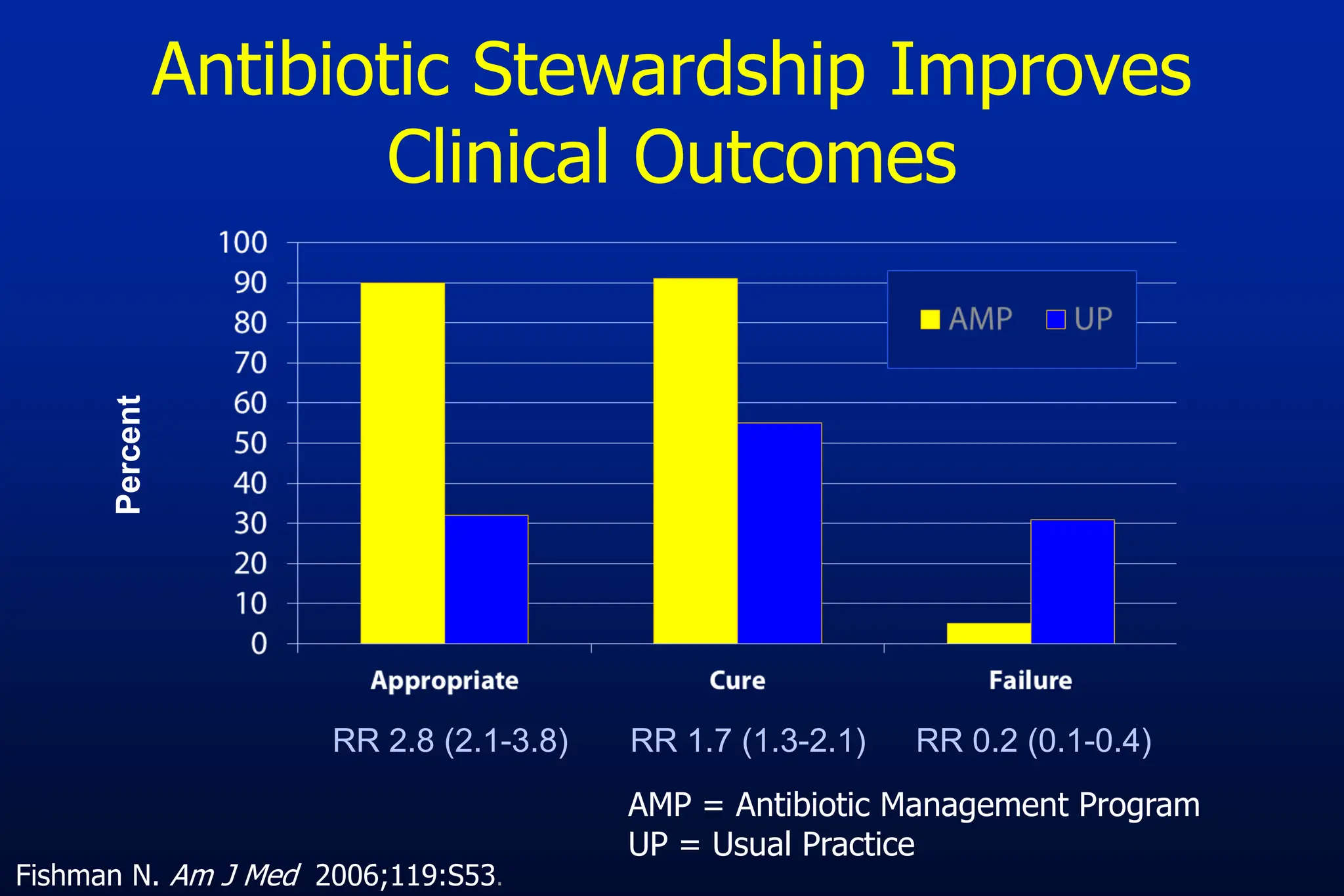
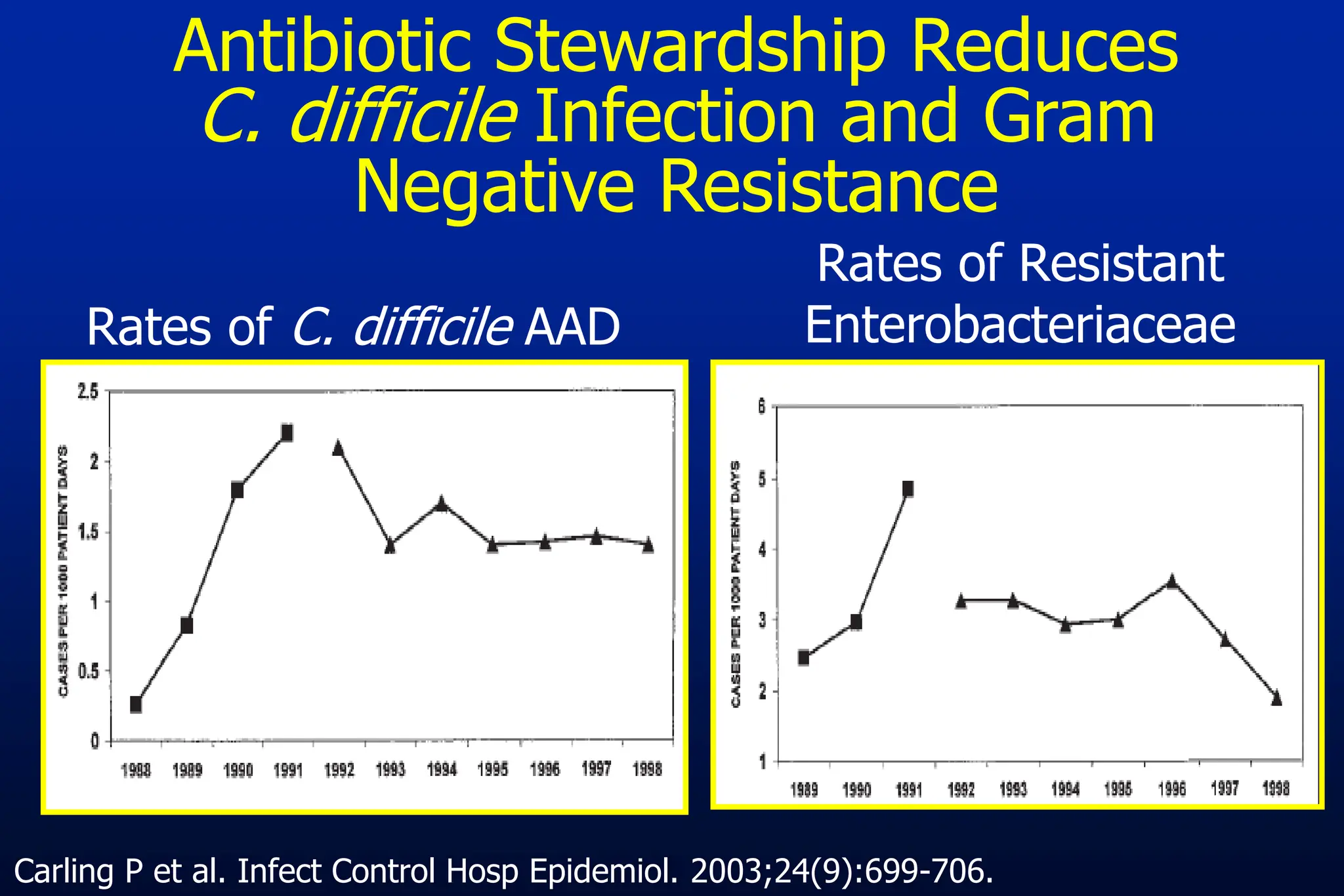
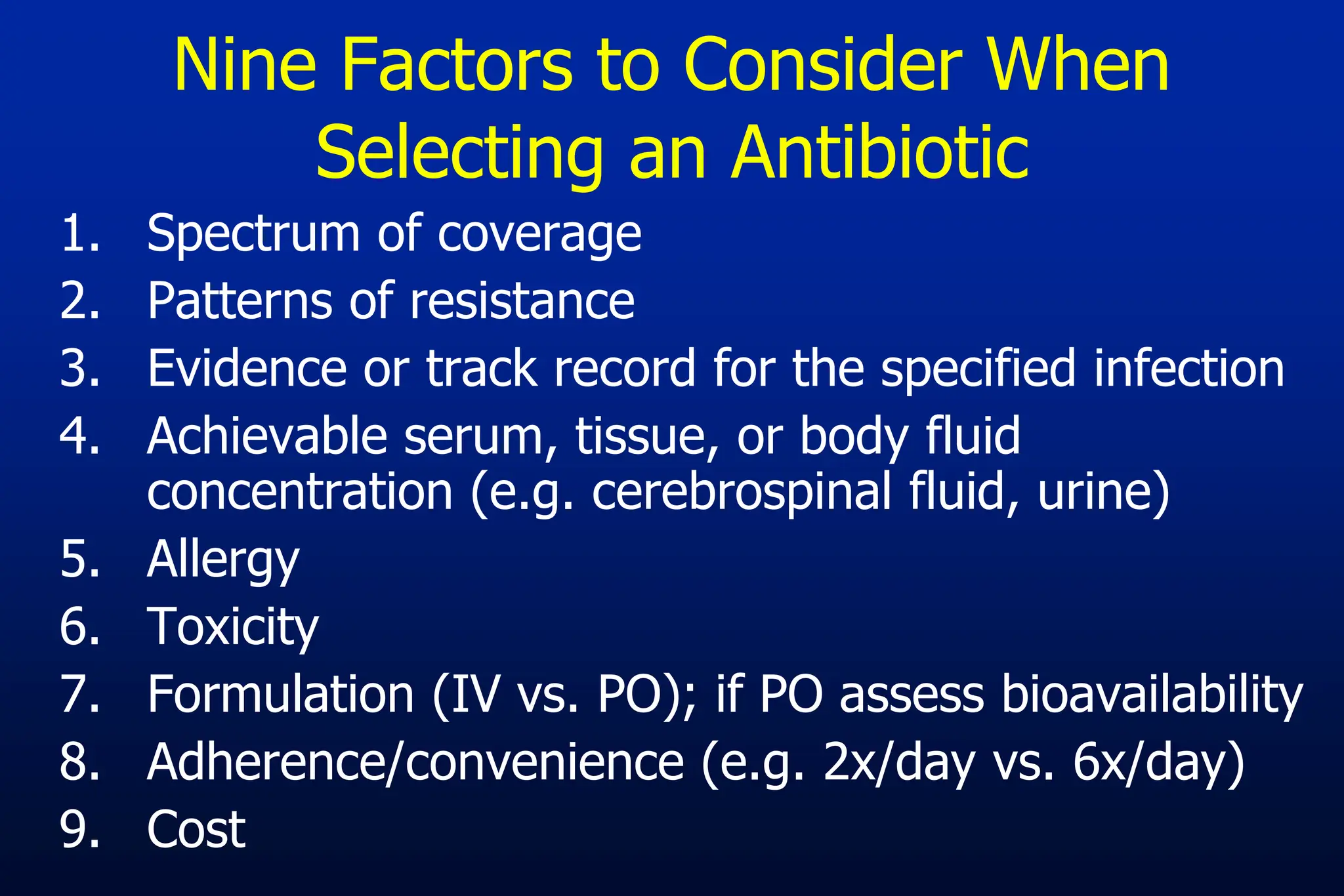
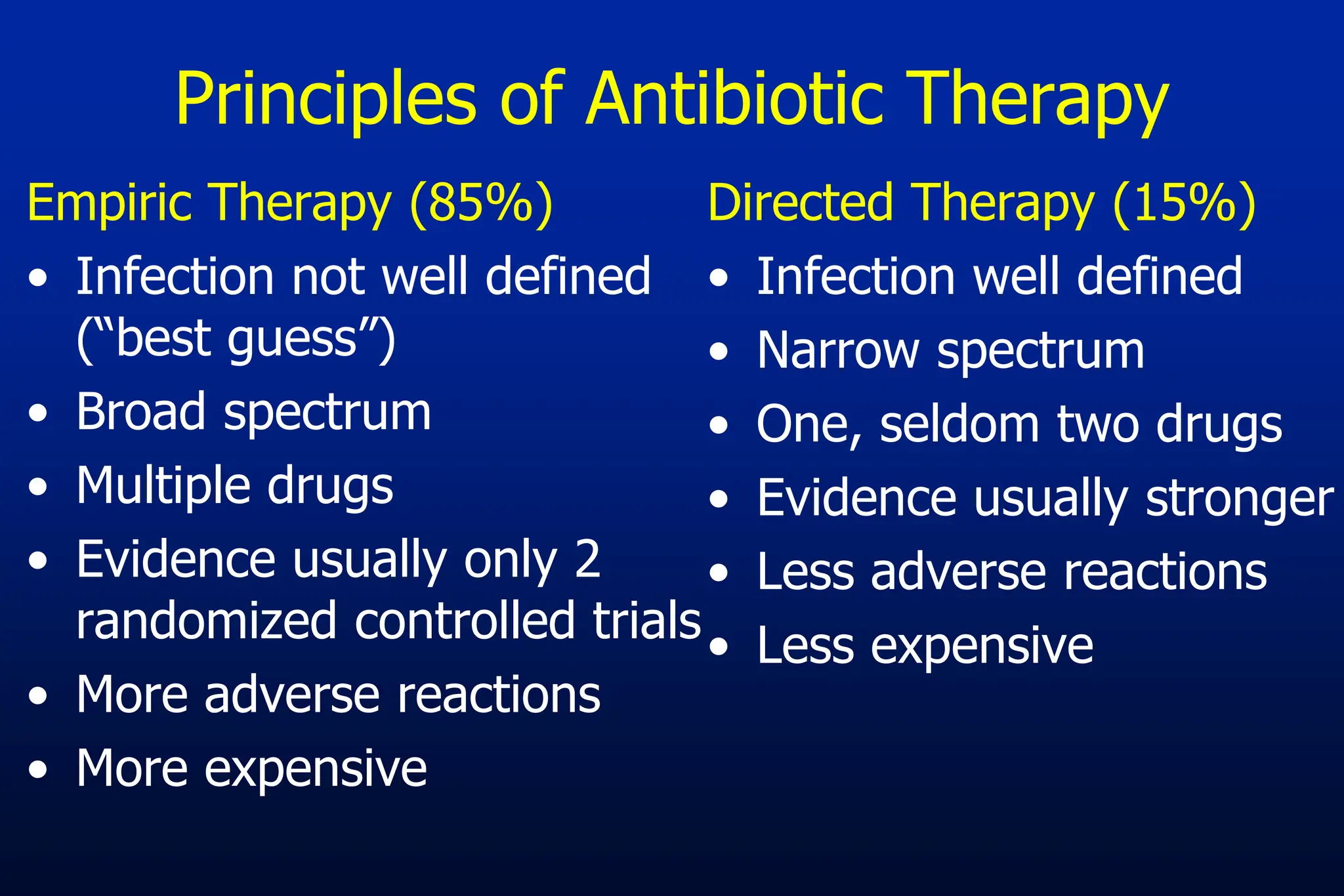
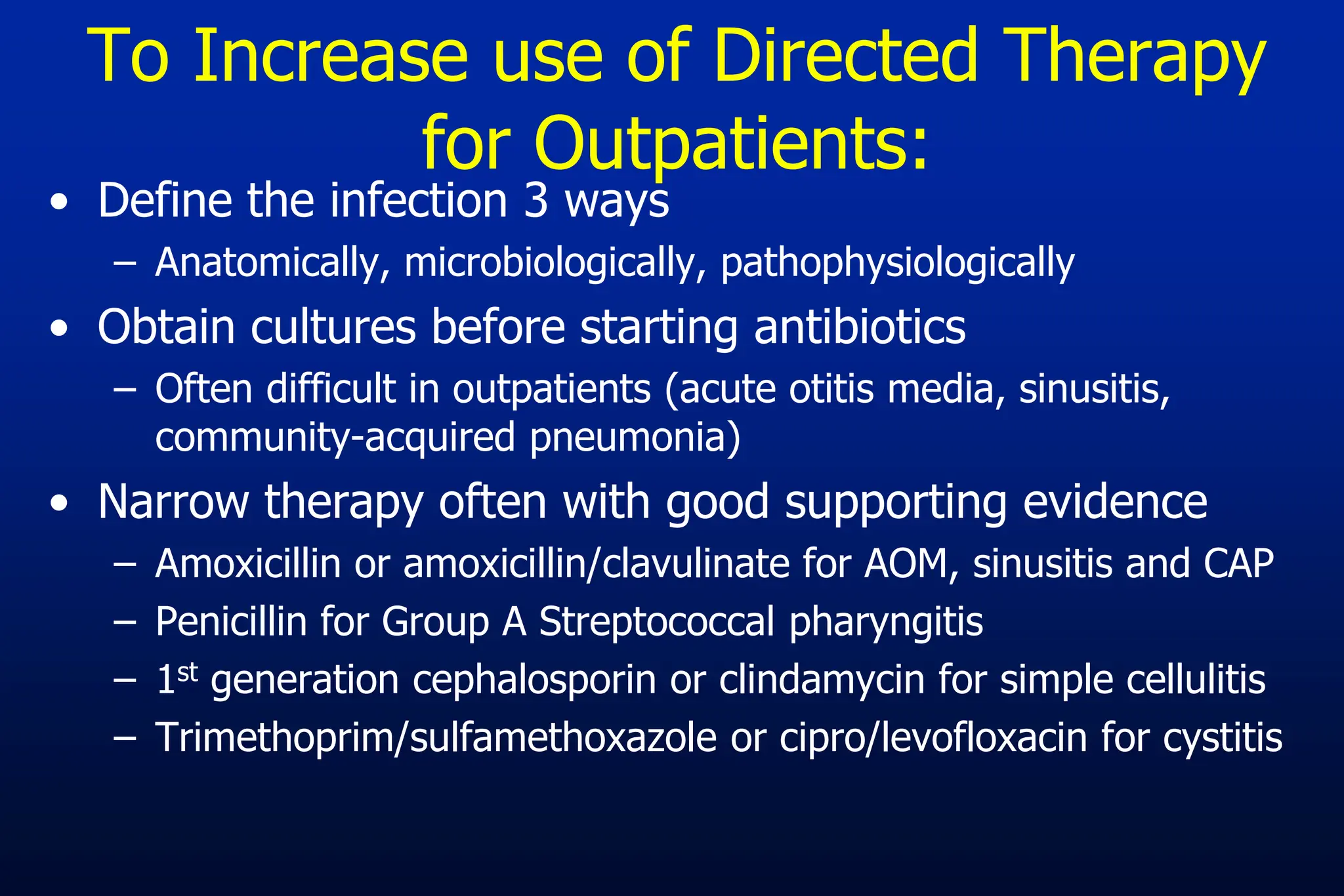
The document outlines an antibiotic stewardship curriculum aimed at promoting prudent antibiotic use, addressing the negative effects of antibiotic overuse, such as resistance and adverse drug events. It emphasizes the importance of appropriate selection and duration of antibiotic therapy, alongside defined tenets for treatment. Key goals include reducing unnecessary use, improving patient outcomes, and limiting antibiotic resistance, with detailed guidance on managing bacterial infections effectively.