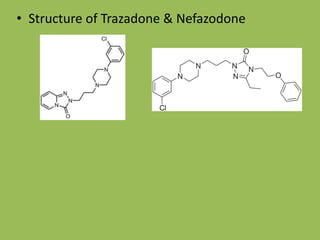
The document describes various classes of antidepressant drugs, including:
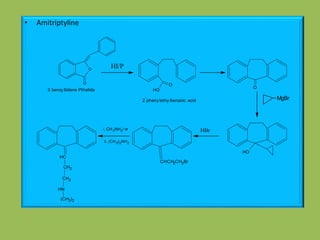
1. Tricyclic antidepressants (TCAs) like amitriptyline
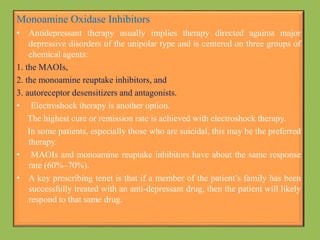
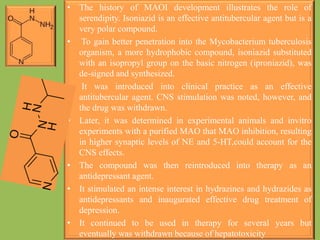
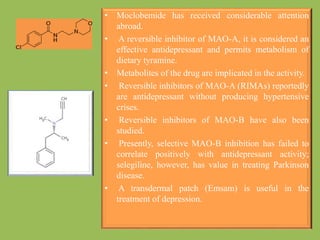
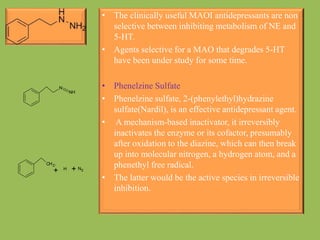
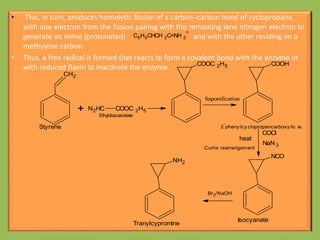
2. Monoamine oxidase inhibitors (MAOIs) like phenelzine that irreversibly inhibit the MAO enzyme
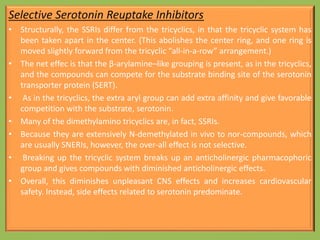
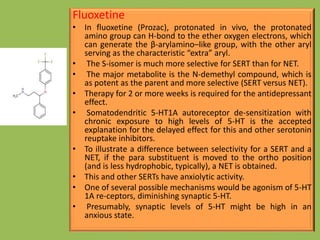
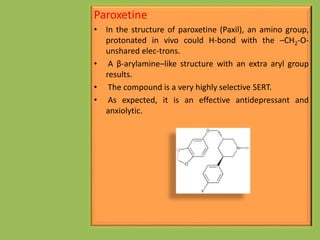
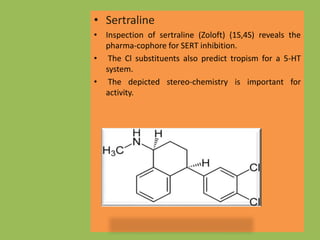
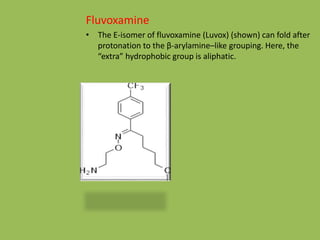
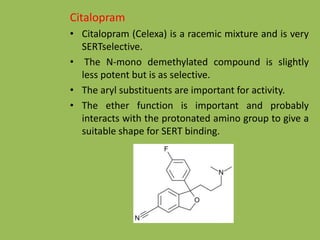
3. Selective serotonin reuptake inhibitors (SSRIs) like fluoxetine that selectively inhibit reuptake of serotonin
It also discusses the biological factors and mechanisms of depression, such as genetic and biochemical influences on neurotransmitter systems in the brain.













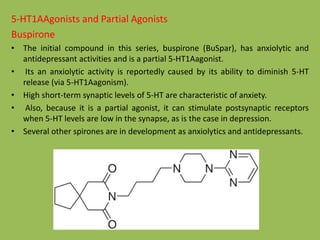
![Synthesis of Buspirone
N
N
Cl
2
-
Chloro pyrimidine
NHNH
N
N
NNH
Cl(CH2)3CN
4-
Chloro butyronitrile
(alkylation)
N
N
N
N
NC
LiAlH4[H]
N
N
N
N
NH2
O
O
O
Spirocyclic glutaric anhydrideO
O
N
N
N
N
N
Buspirone](https://image.slidesharecdn.com/antidepressants-180612082811/85/Antidepressants-32-320.jpg)
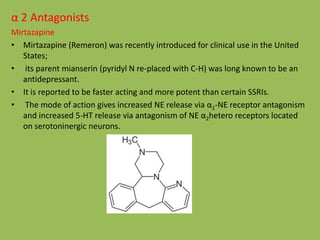
![O
CH3
Acetophenone
1) HCHO
2) (CH3)2NH
Mannich reaction
O N
CH3
CH3
[H]
B2H6
Reduction
OH N
CH3
CH3
SOCl 2
Cl N
CH3
CH3
OH
CF3
NaOH
F3C
O
N
CH3
CH3
CNBr
-CH3
Van Braun reaction
F3C
O
N
H
CH3
Fluxetine
Synthesis of Fluxetine](https://image.slidesharecdn.com/antidepressants-180612082811/85/Antidepressants-34-320.jpg)
