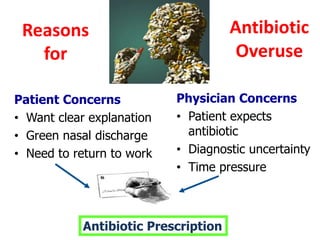
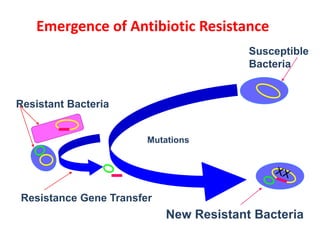
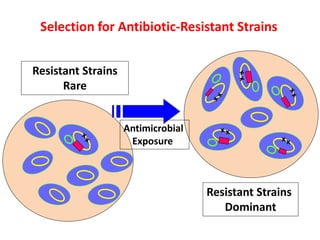
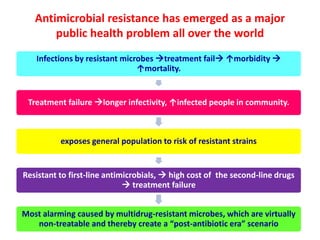
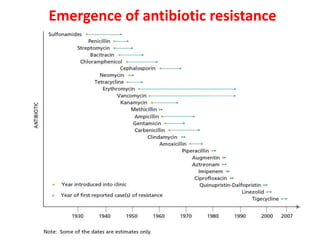
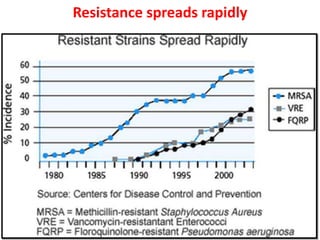
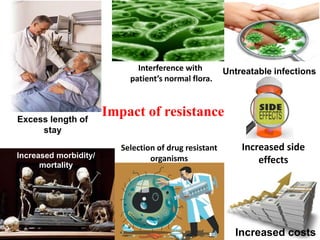
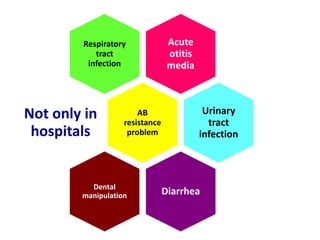
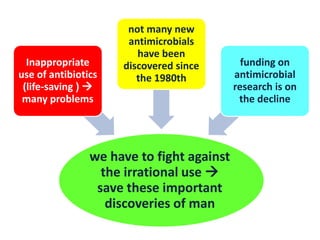
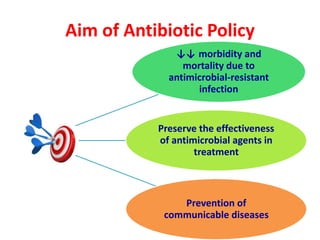
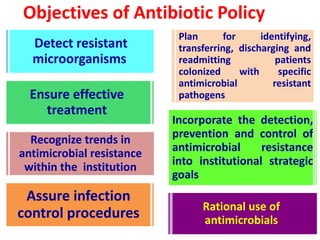
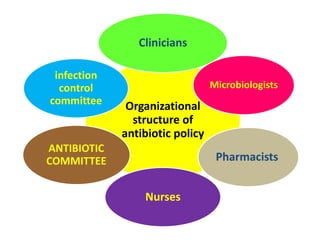
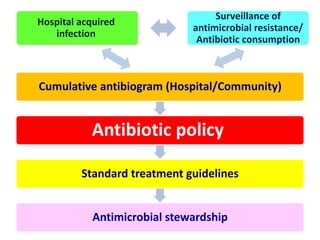
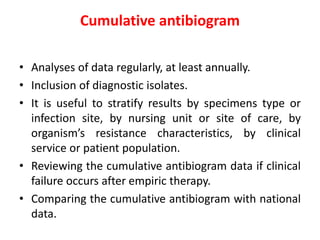
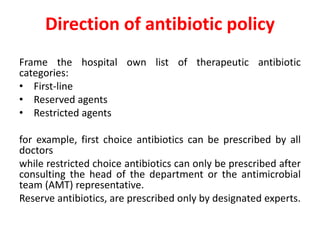
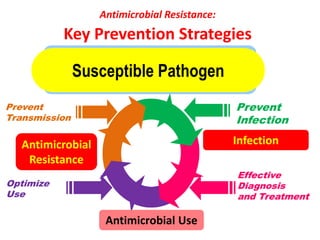
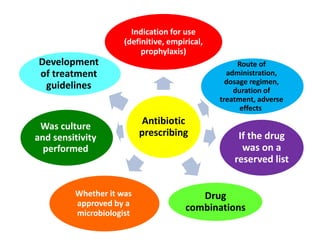
This document discusses rational antibiotic use and antibiotic resistance. It defines rational antibiotic use as patients receiving appropriate medications for their clinical needs in adequate doses and durations at the lowest cost. Antibiotic overuse and misuse can lead to antibiotic resistance where infections become difficult or impossible to treat. The emergence of multidrug-resistant bacteria is a major public health threat. Hospitals should establish antibiotic policies and guidelines to promote appropriate antibiotic prescribing and prevent the spread of resistance.