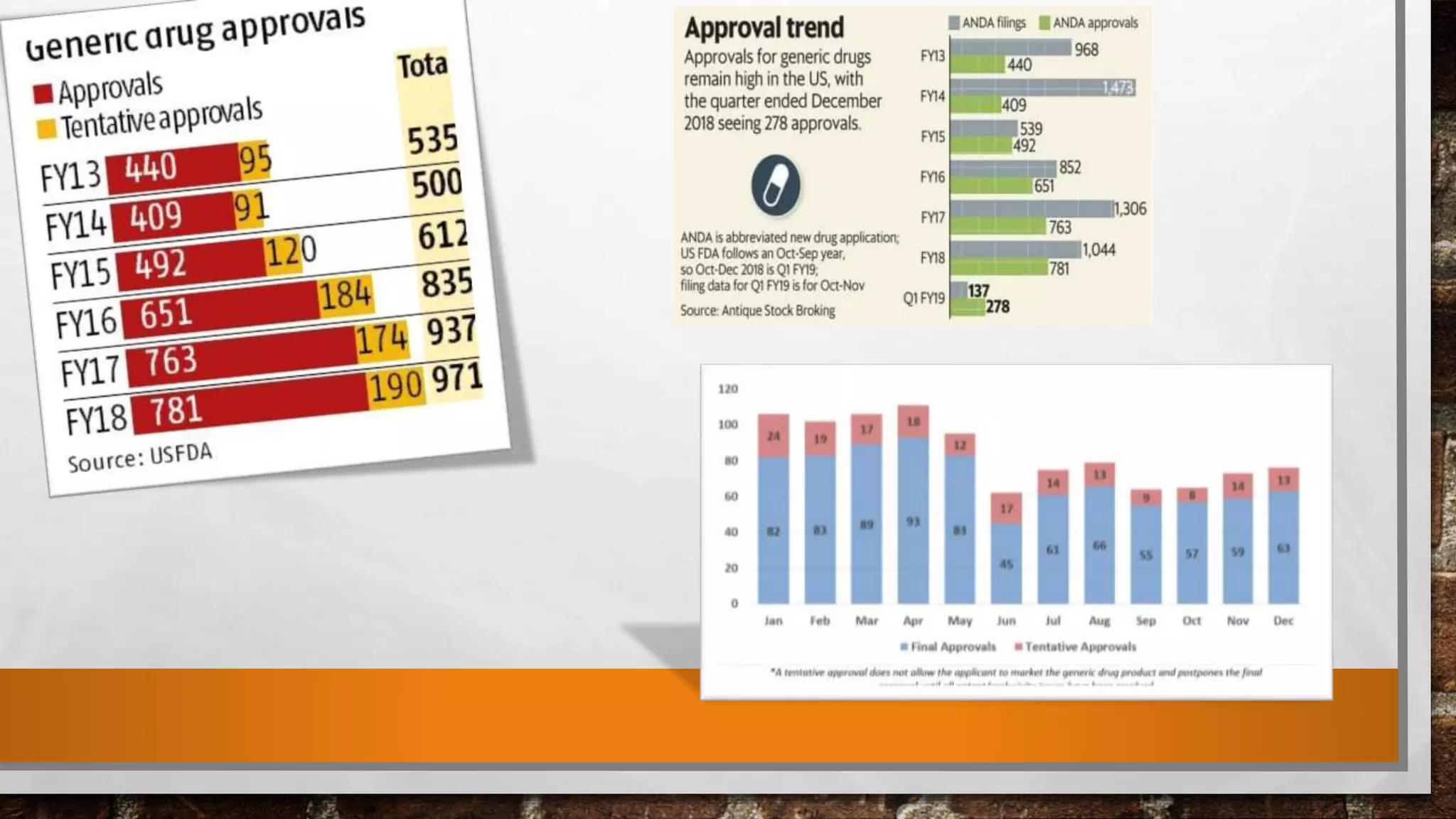
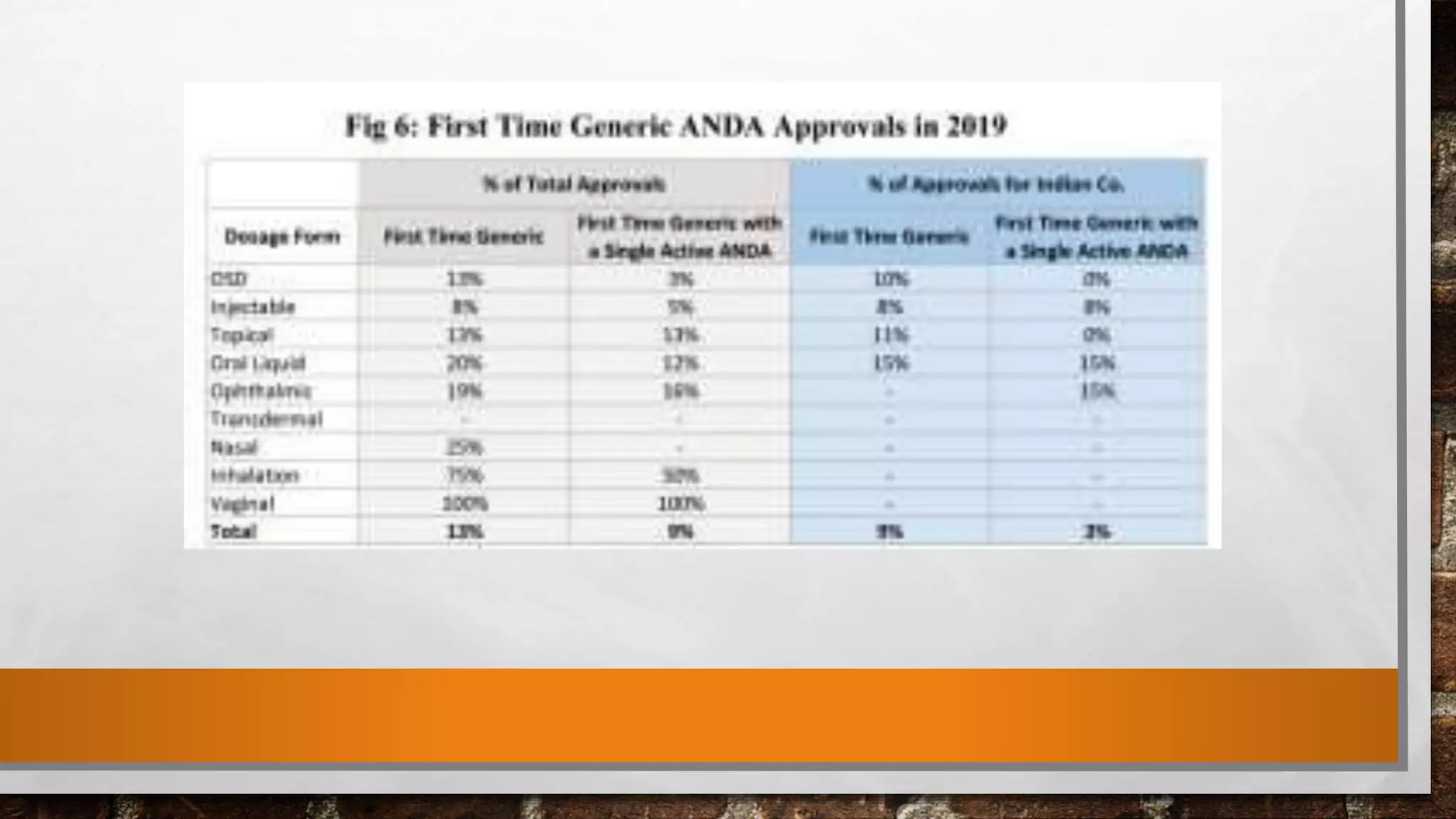
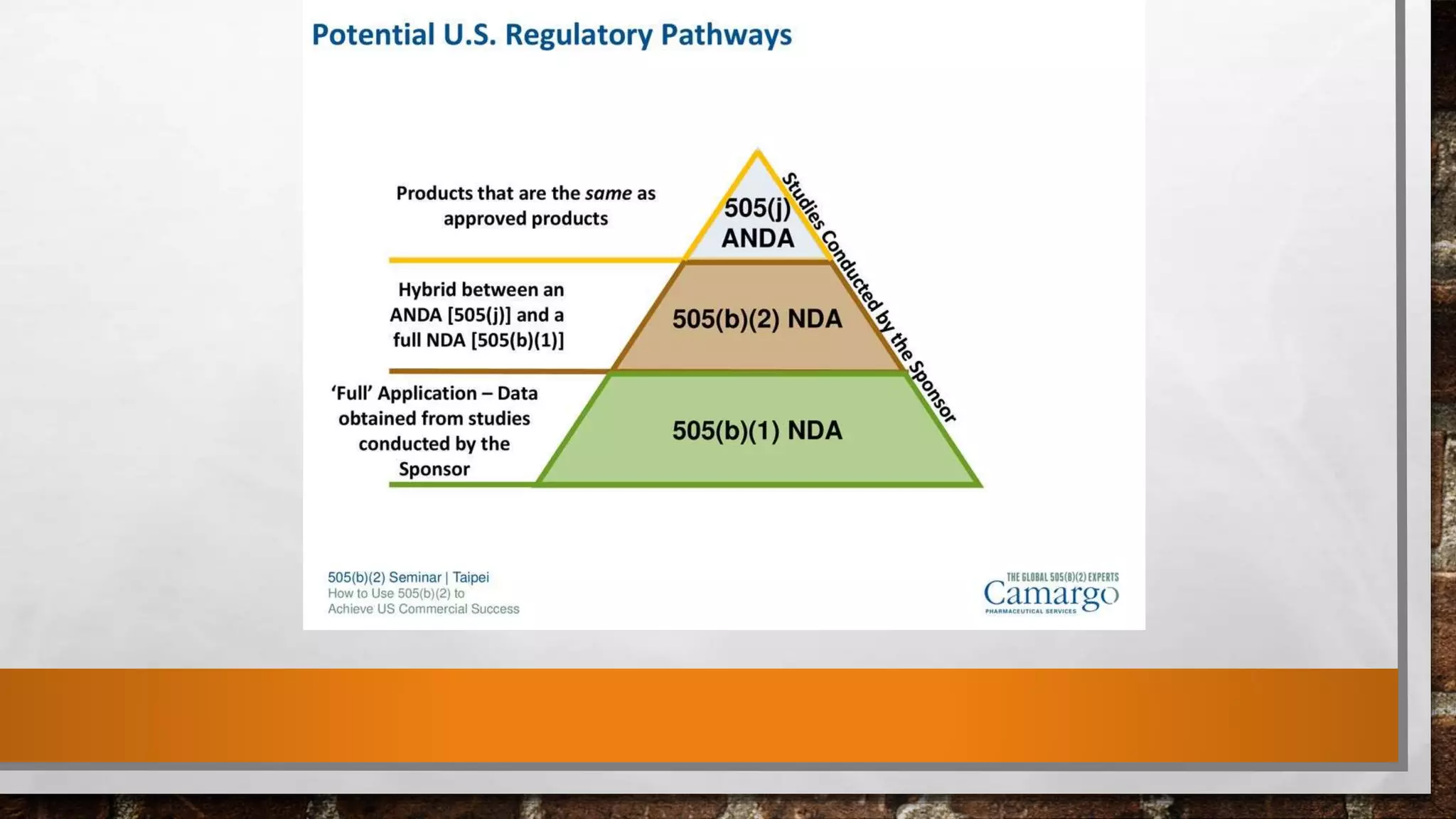
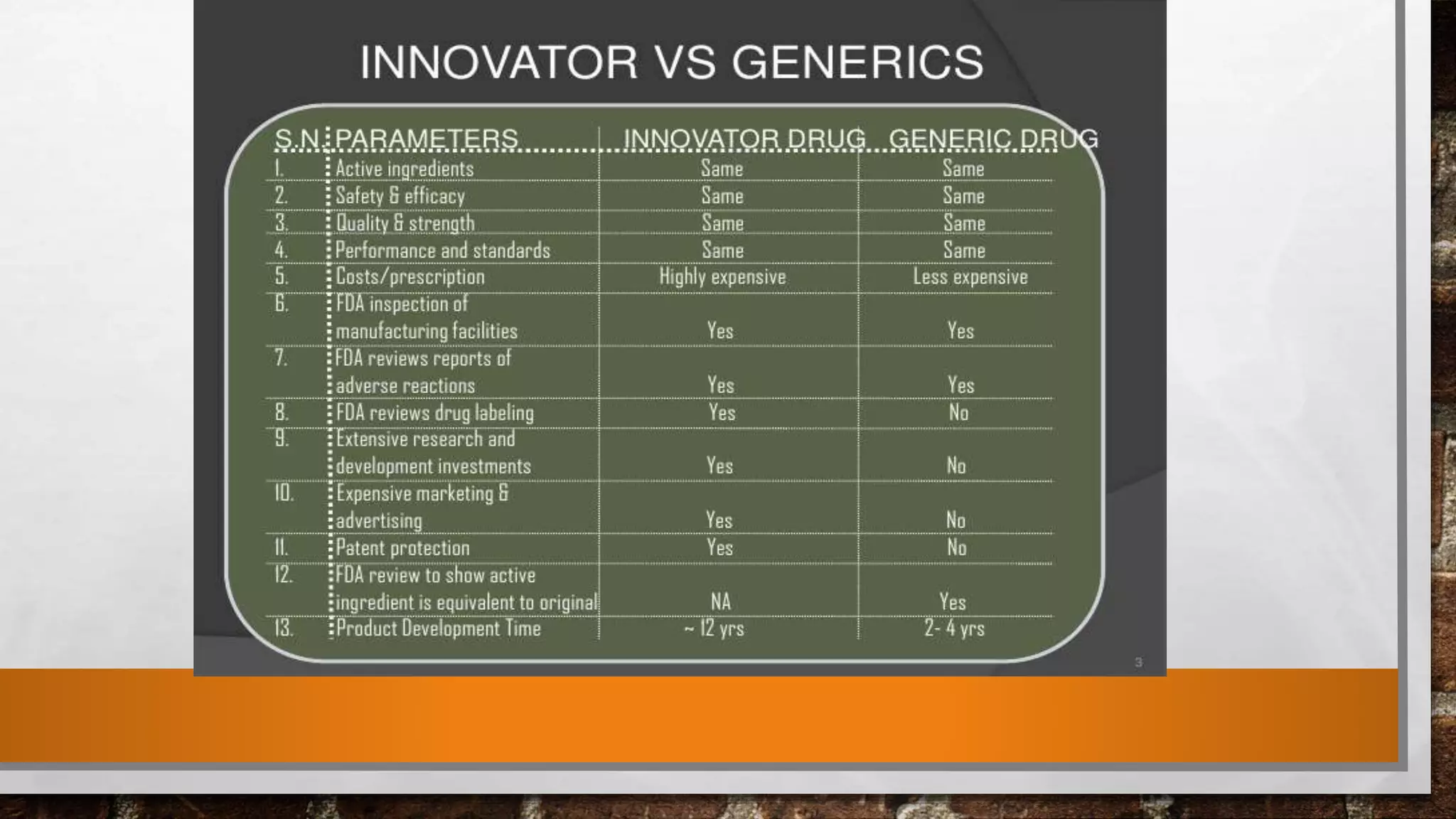
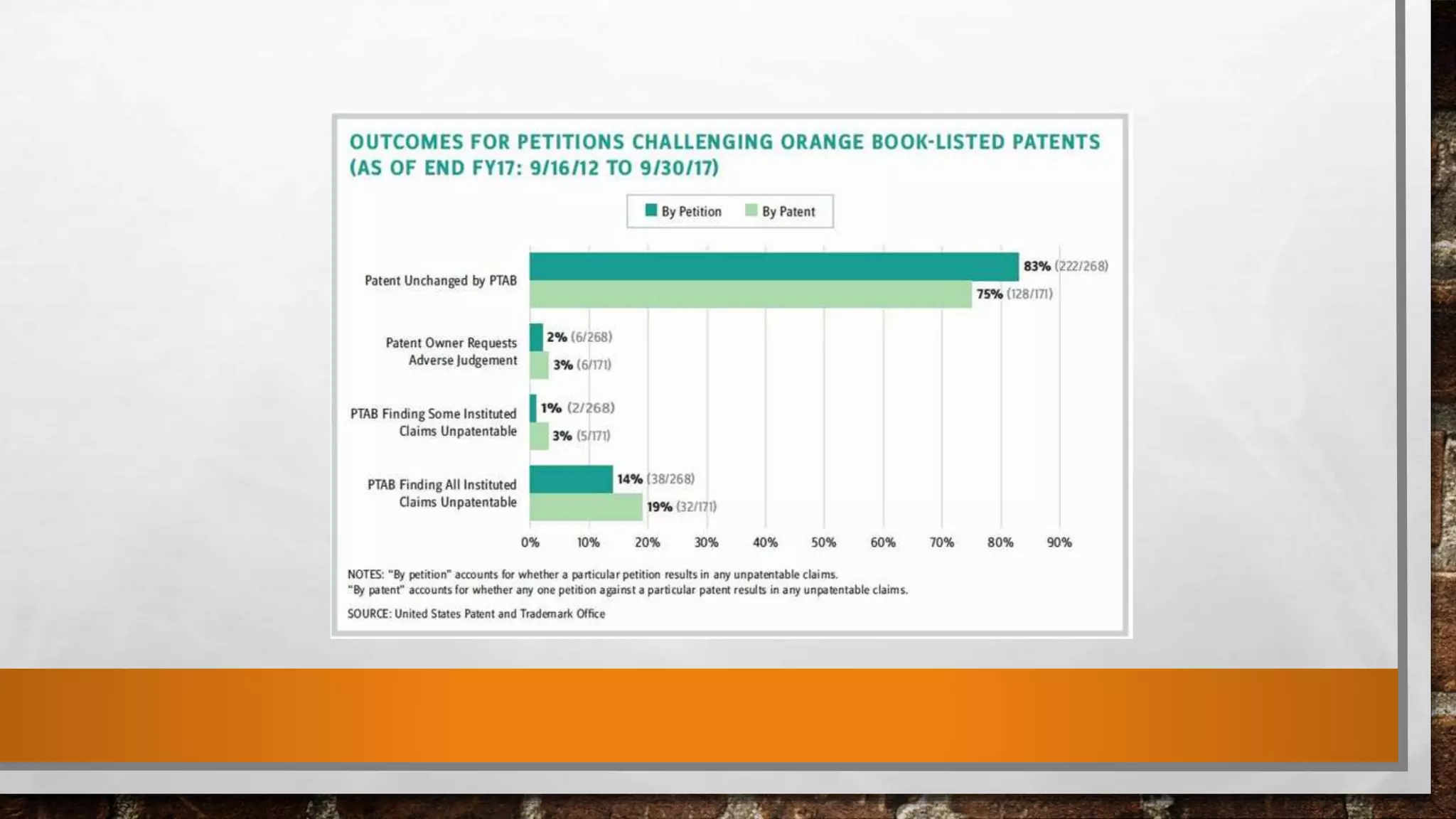
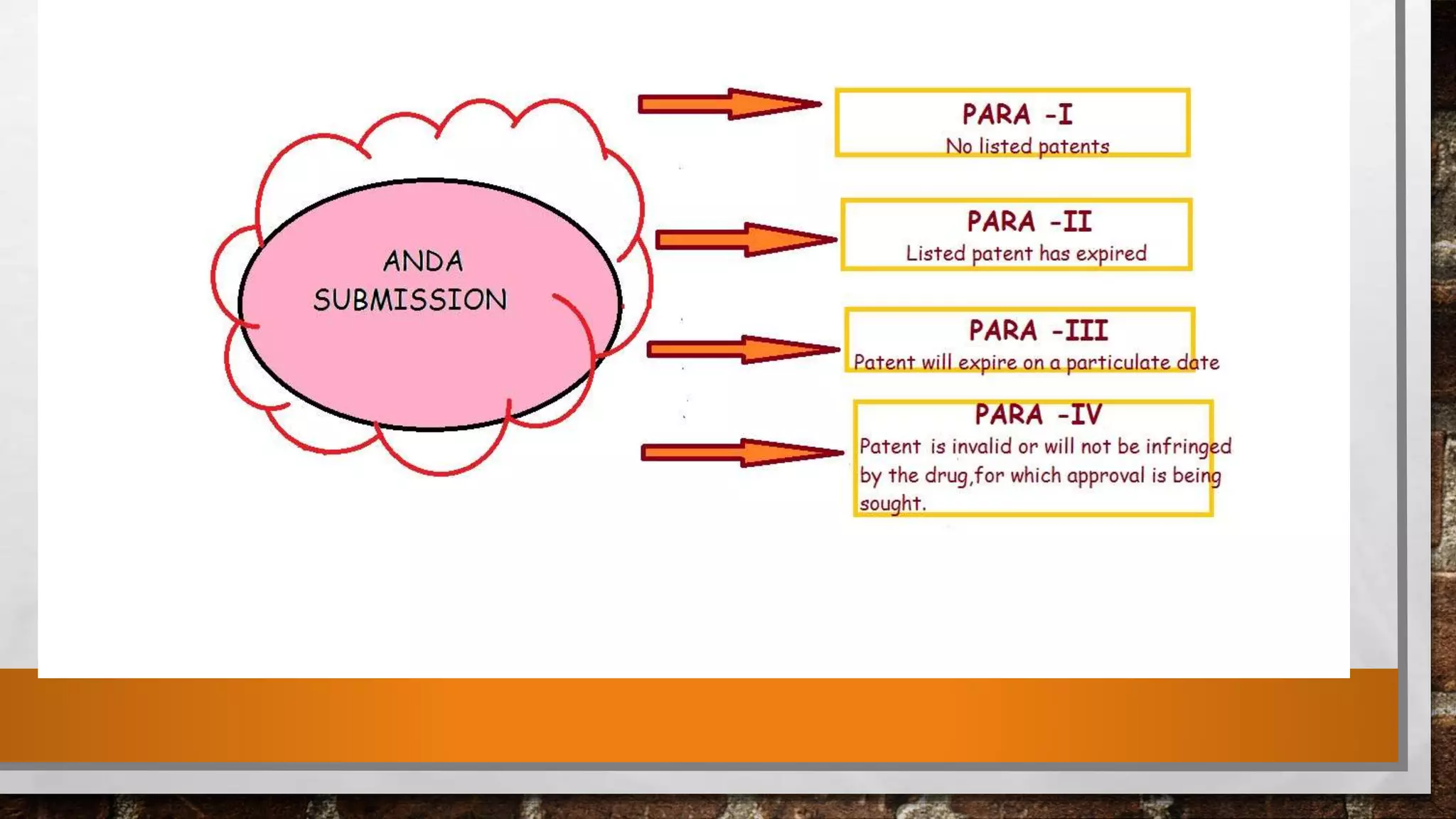
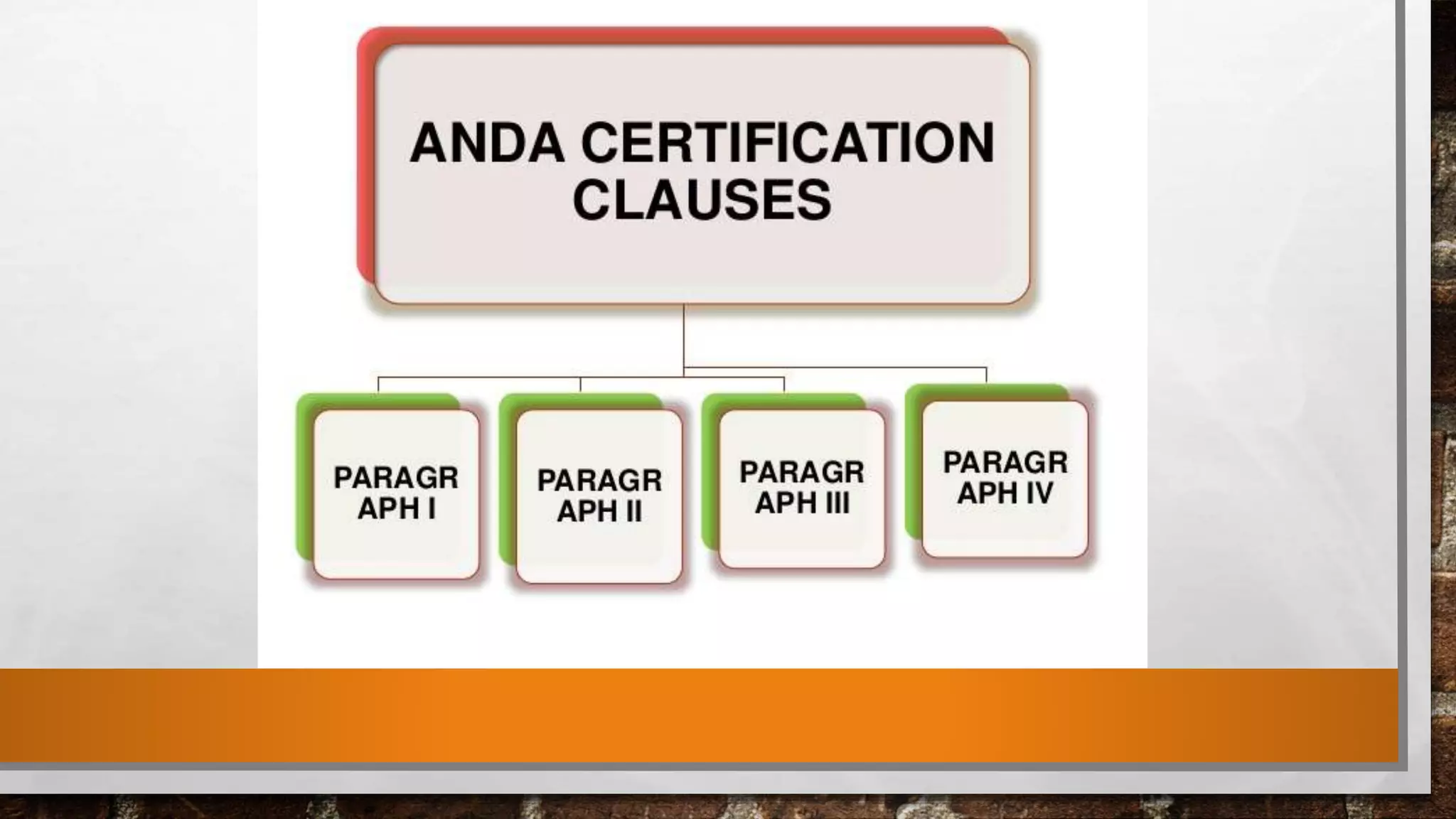
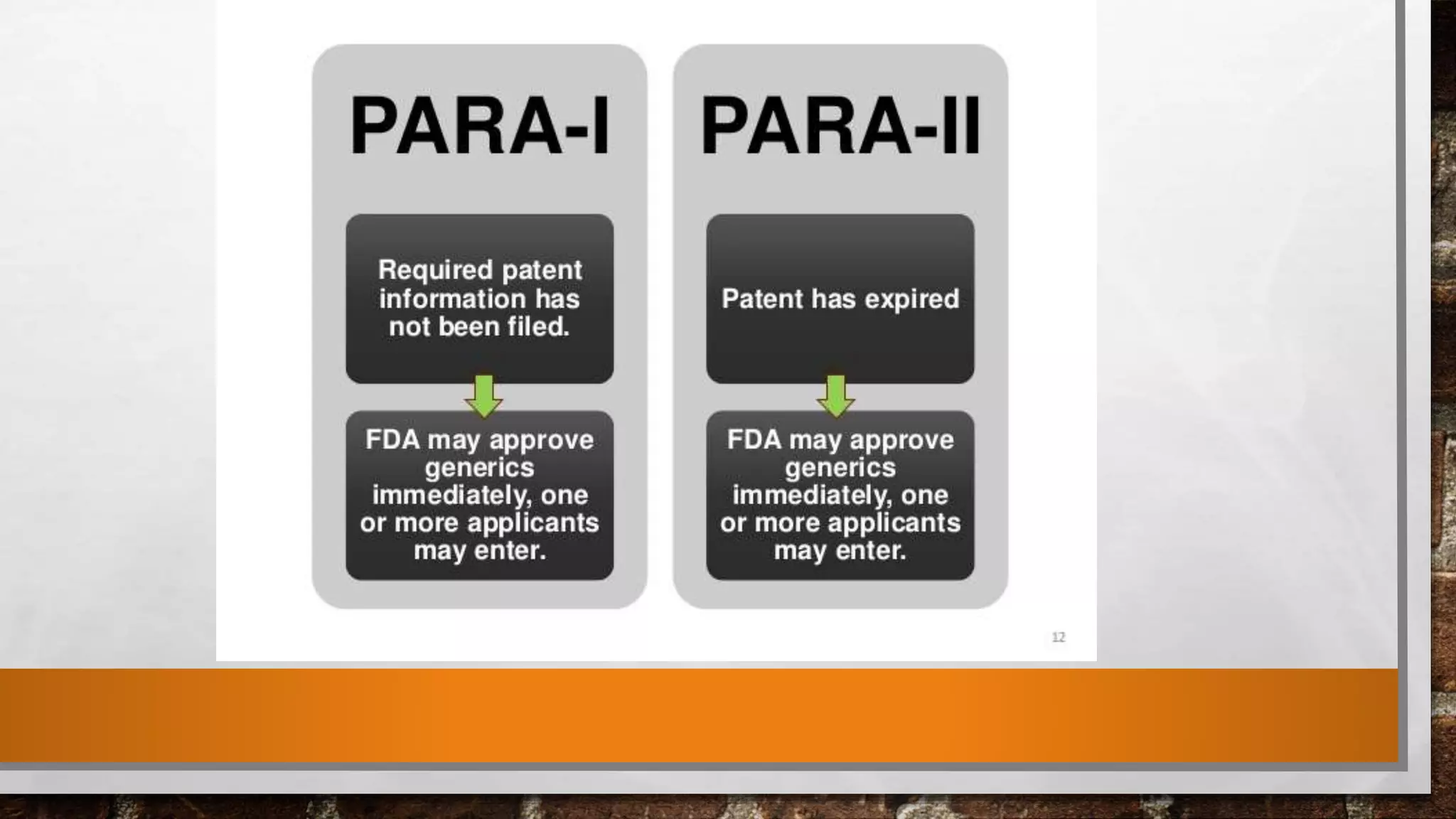
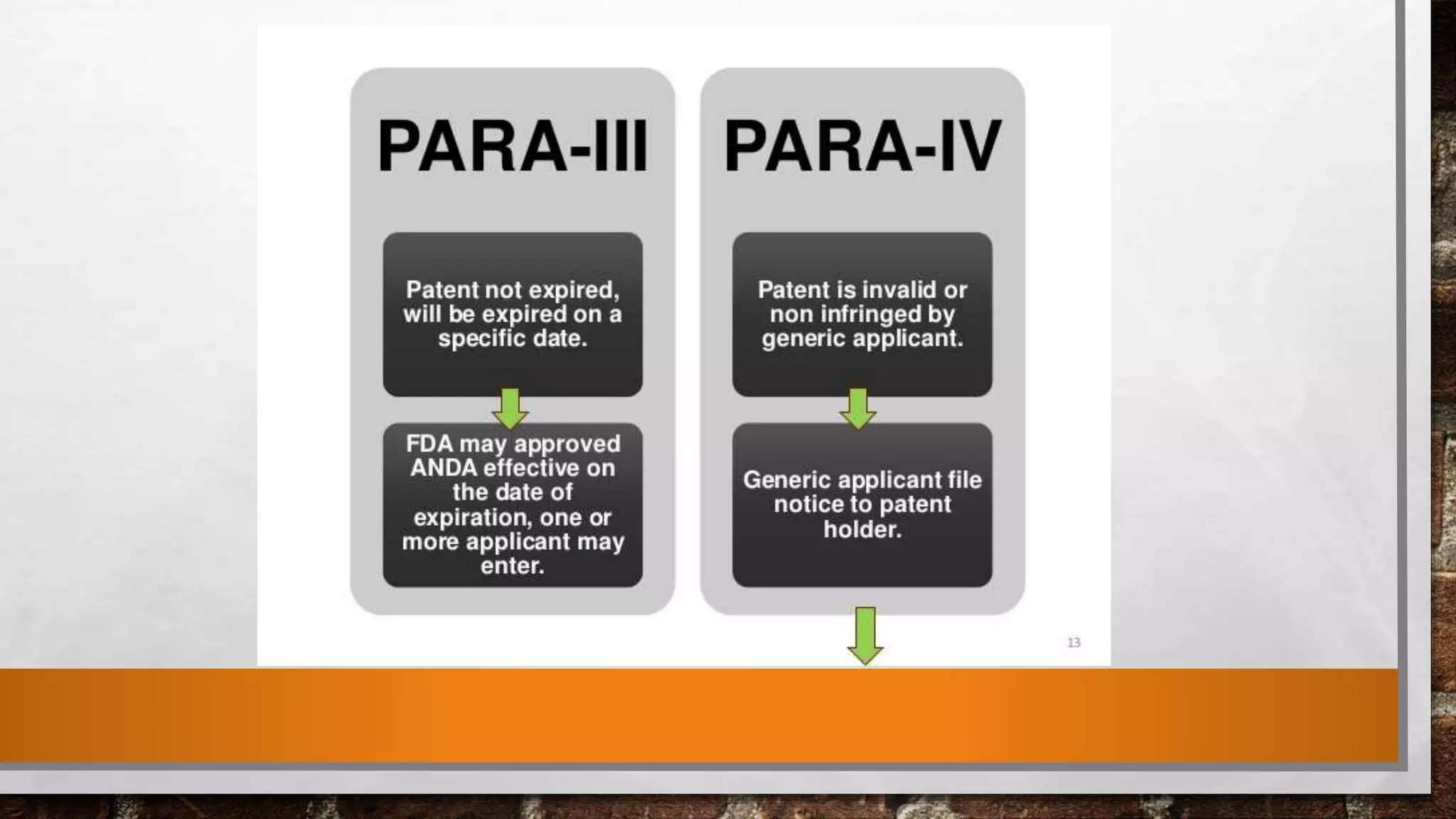
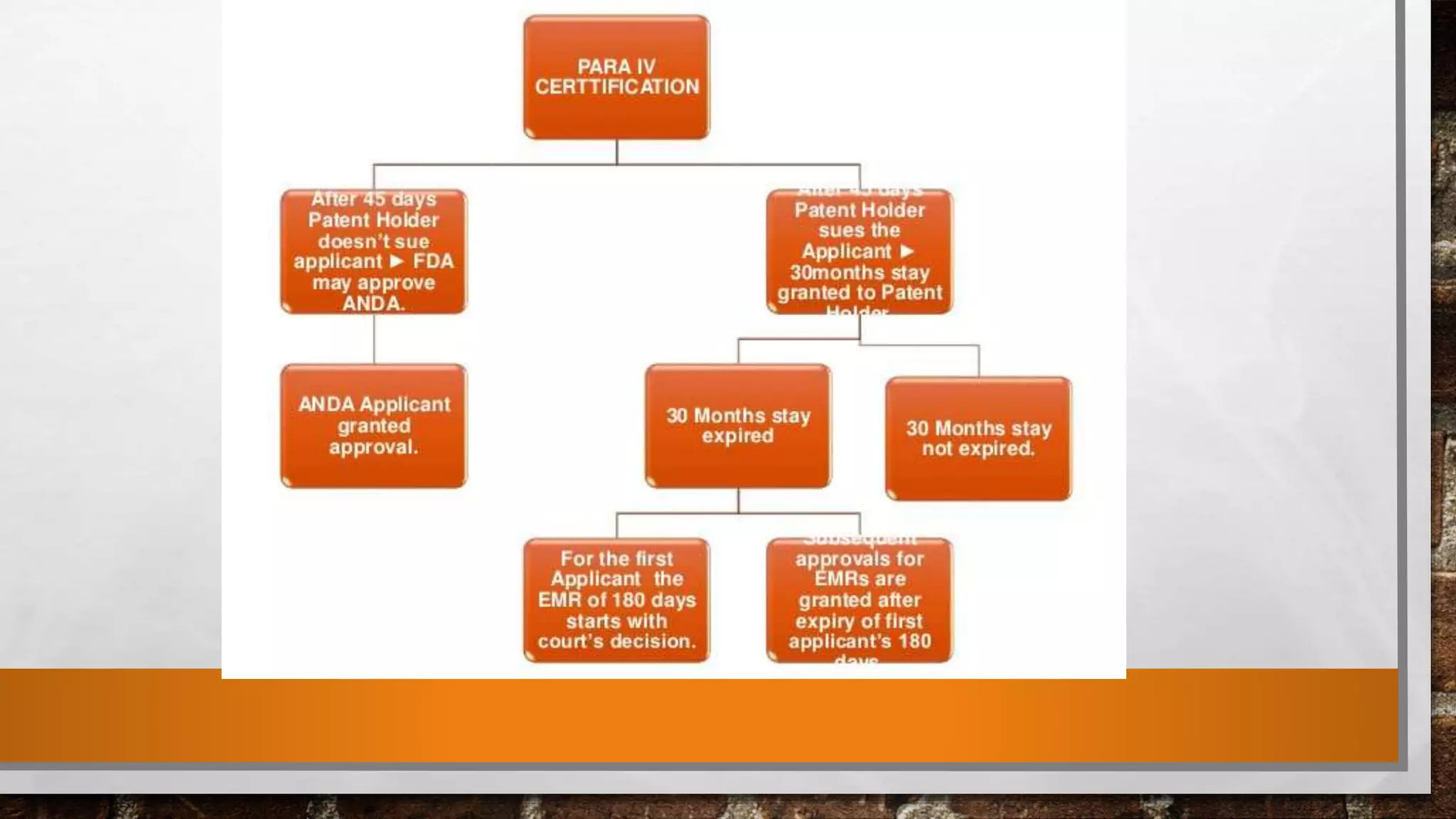
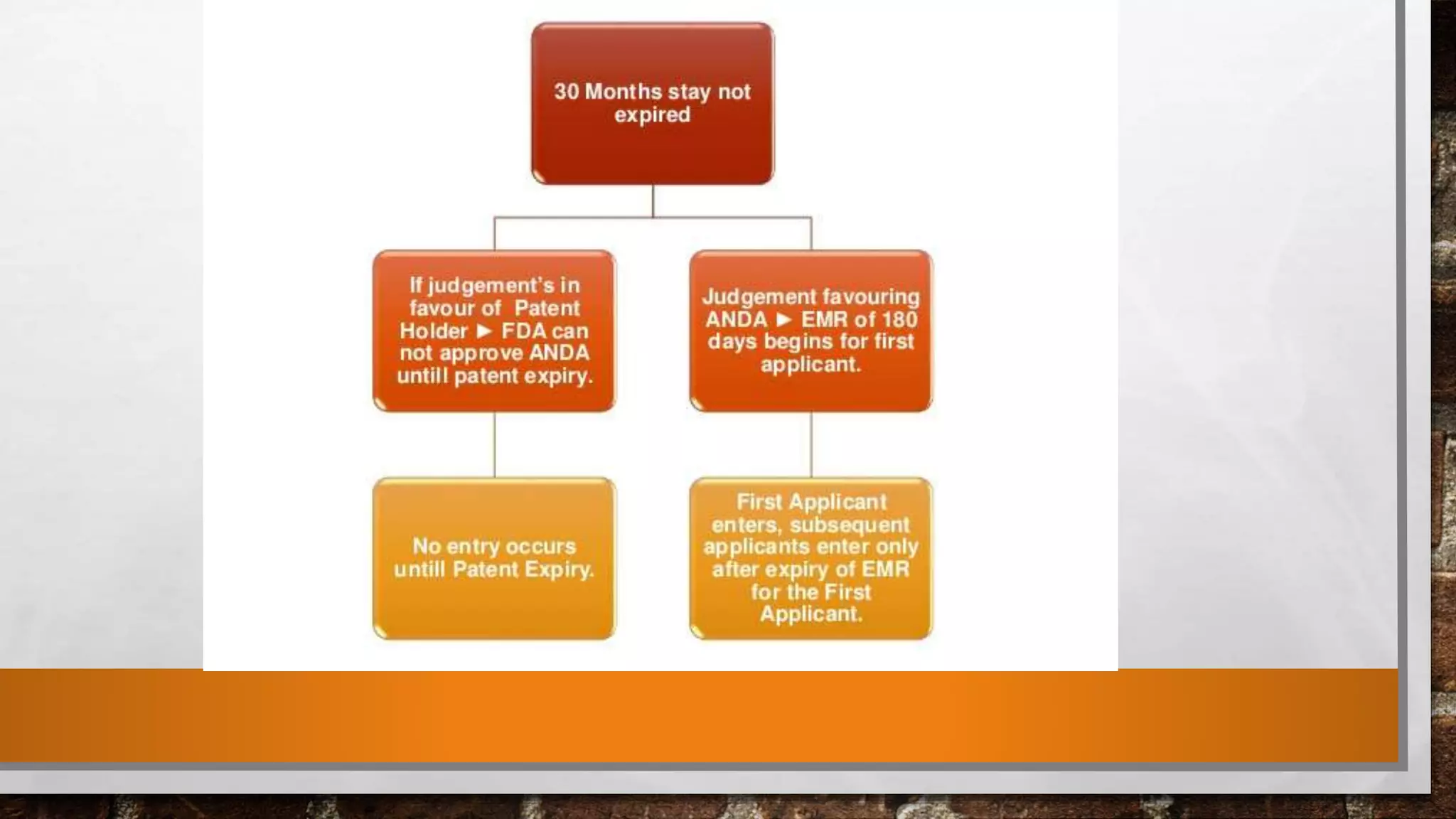
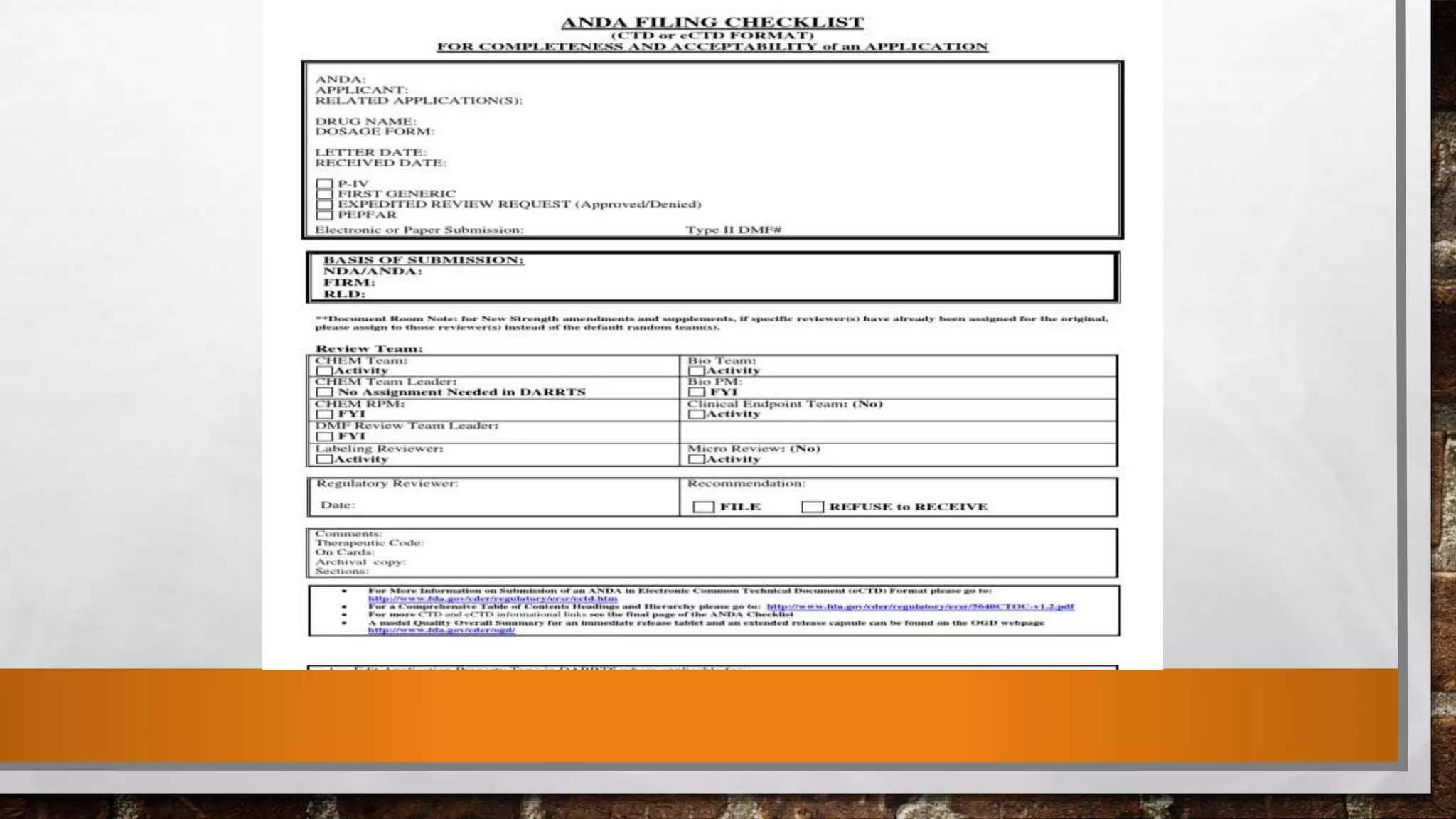
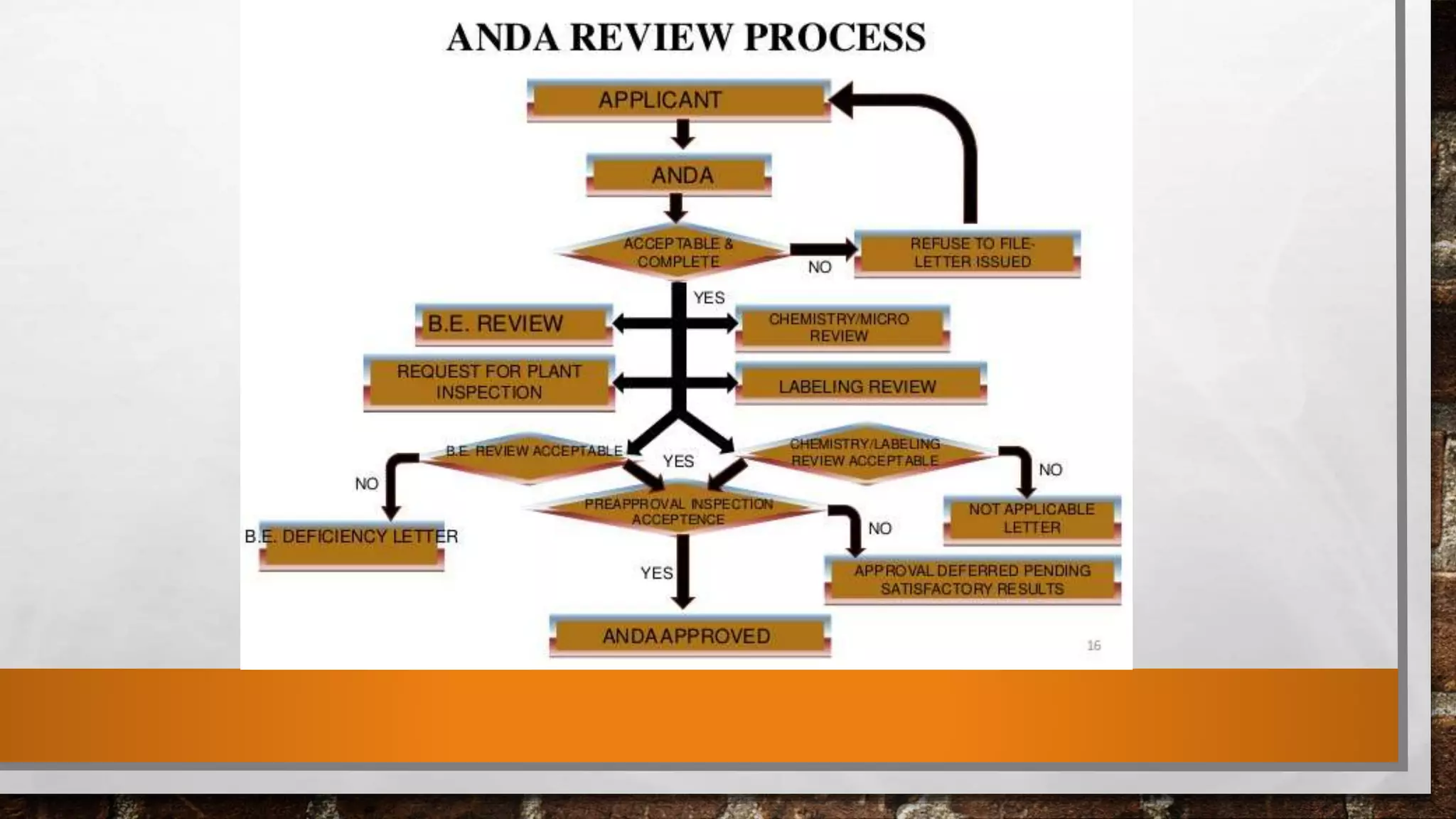
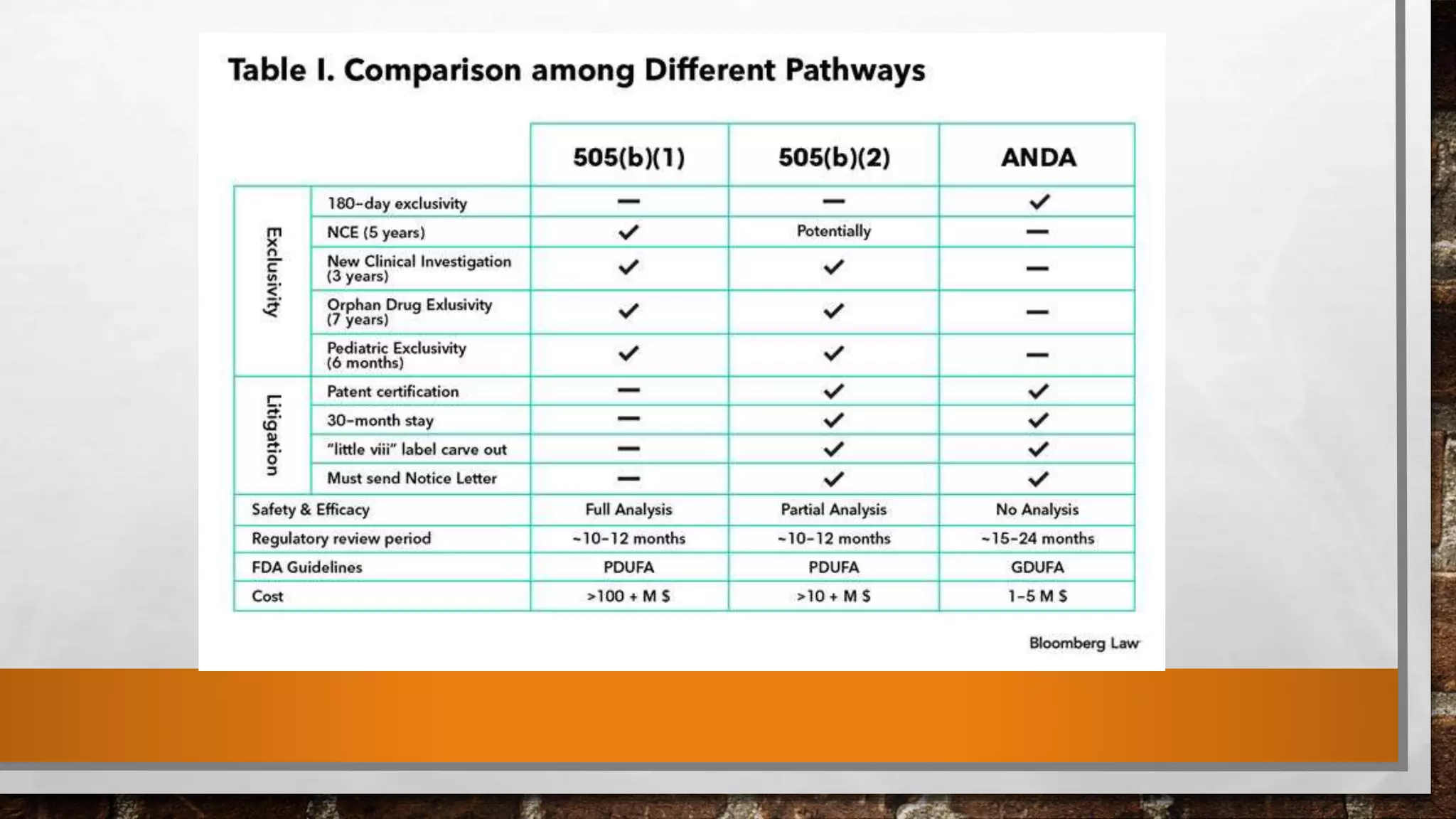
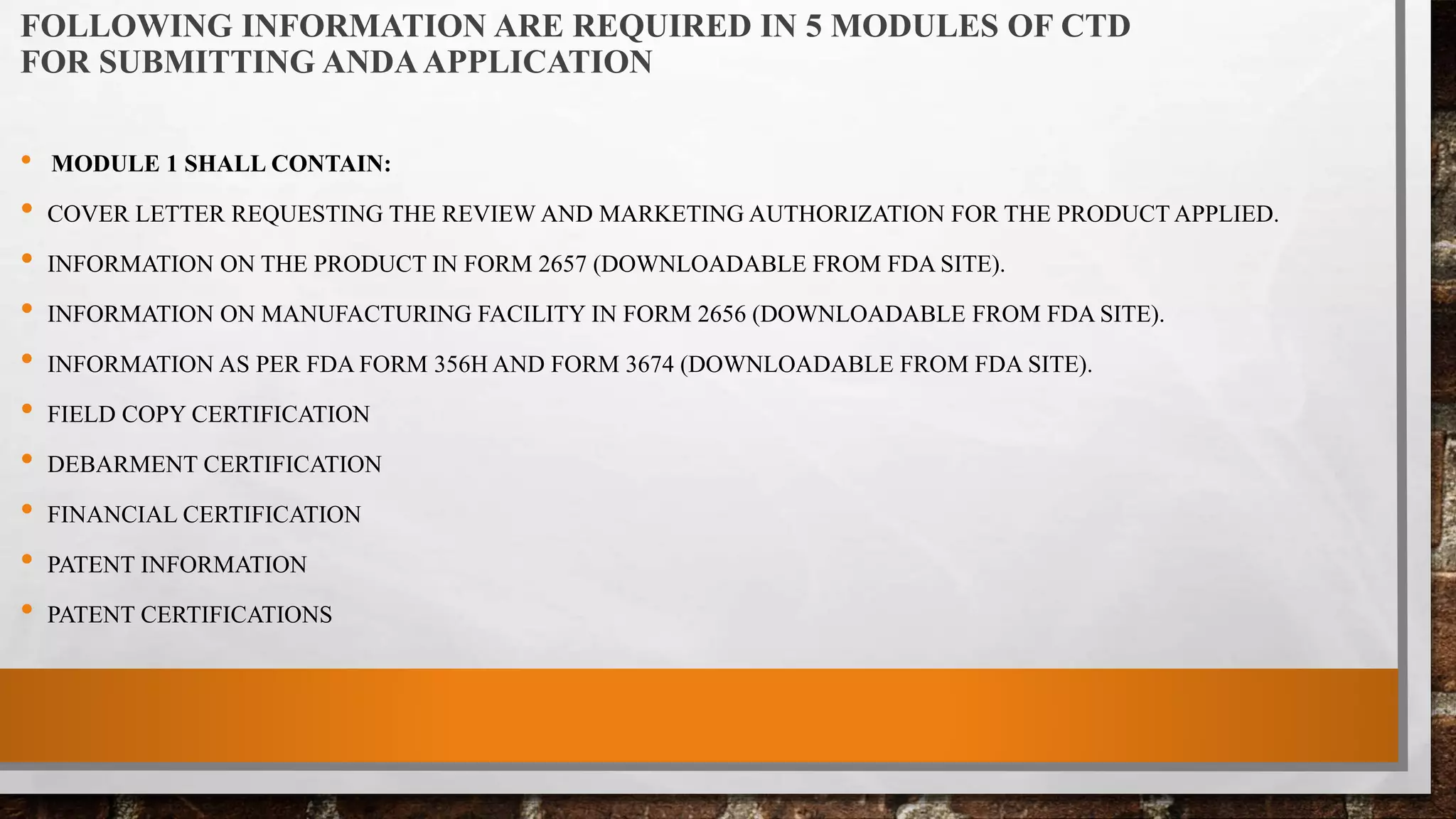
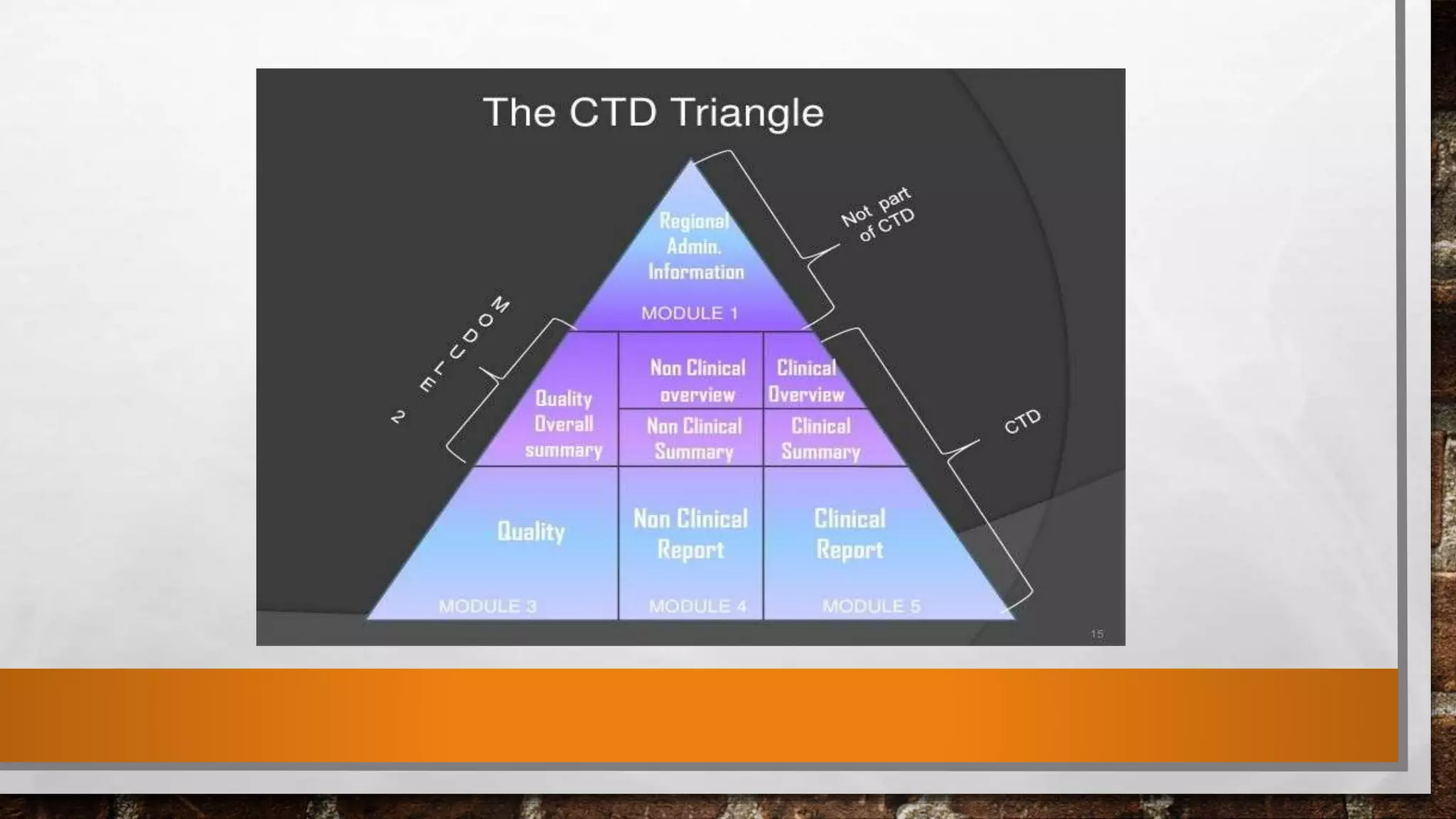
The document discusses the requirements and procedures for submitting an Abbreviated New Drug Application (ANDA) under Paragraph IV certification, which allows generic drugs to be marketed before the expiration of brand-name patents. It outlines the necessary modules, submission formats, and regulatory hurdles faced by applicants, especially with the shift to electronic submissions only. The conclusion emphasizes the increasing challenges since 2000, despite the growing number of companies entering the Paragraph IV market.