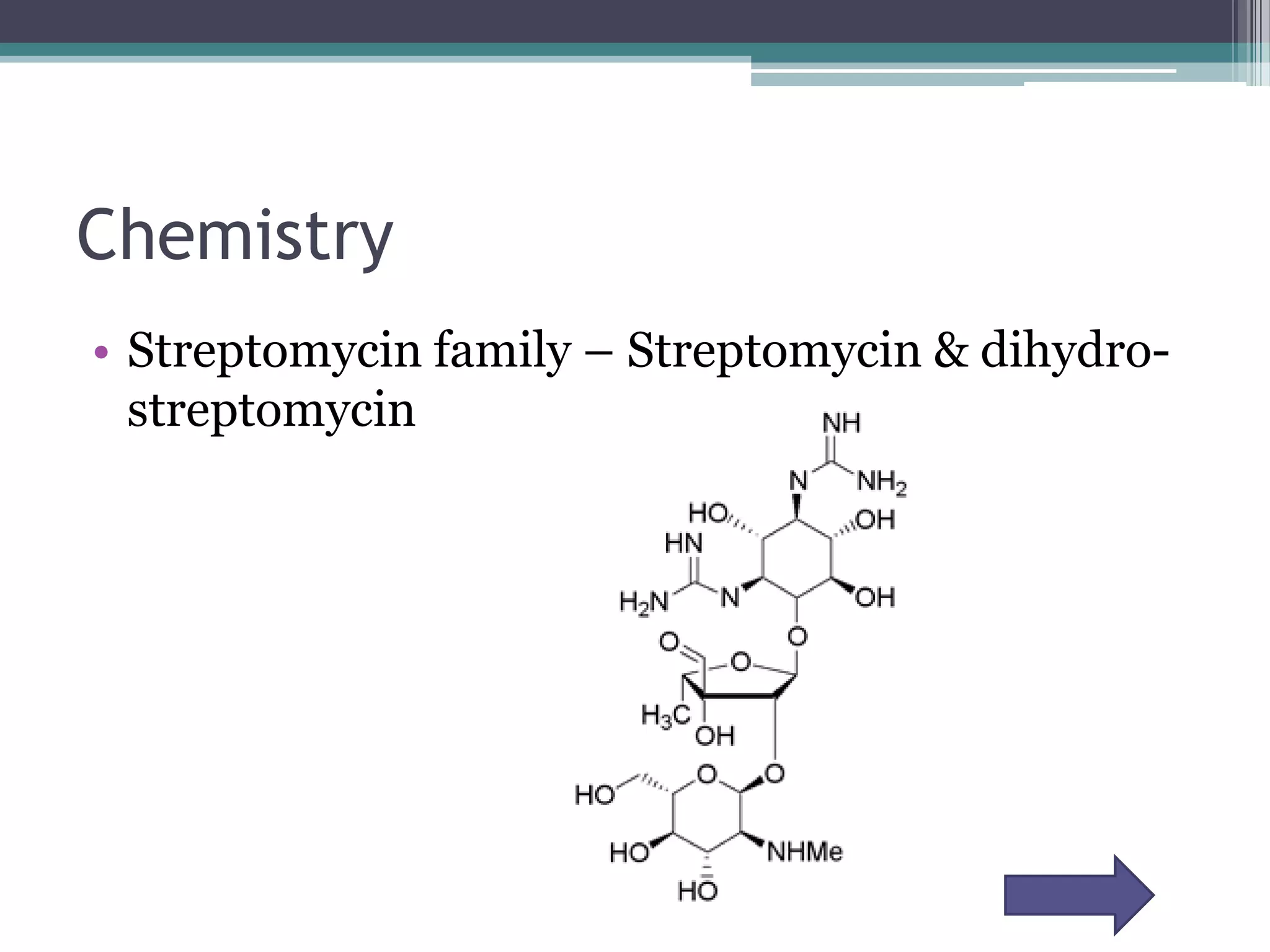
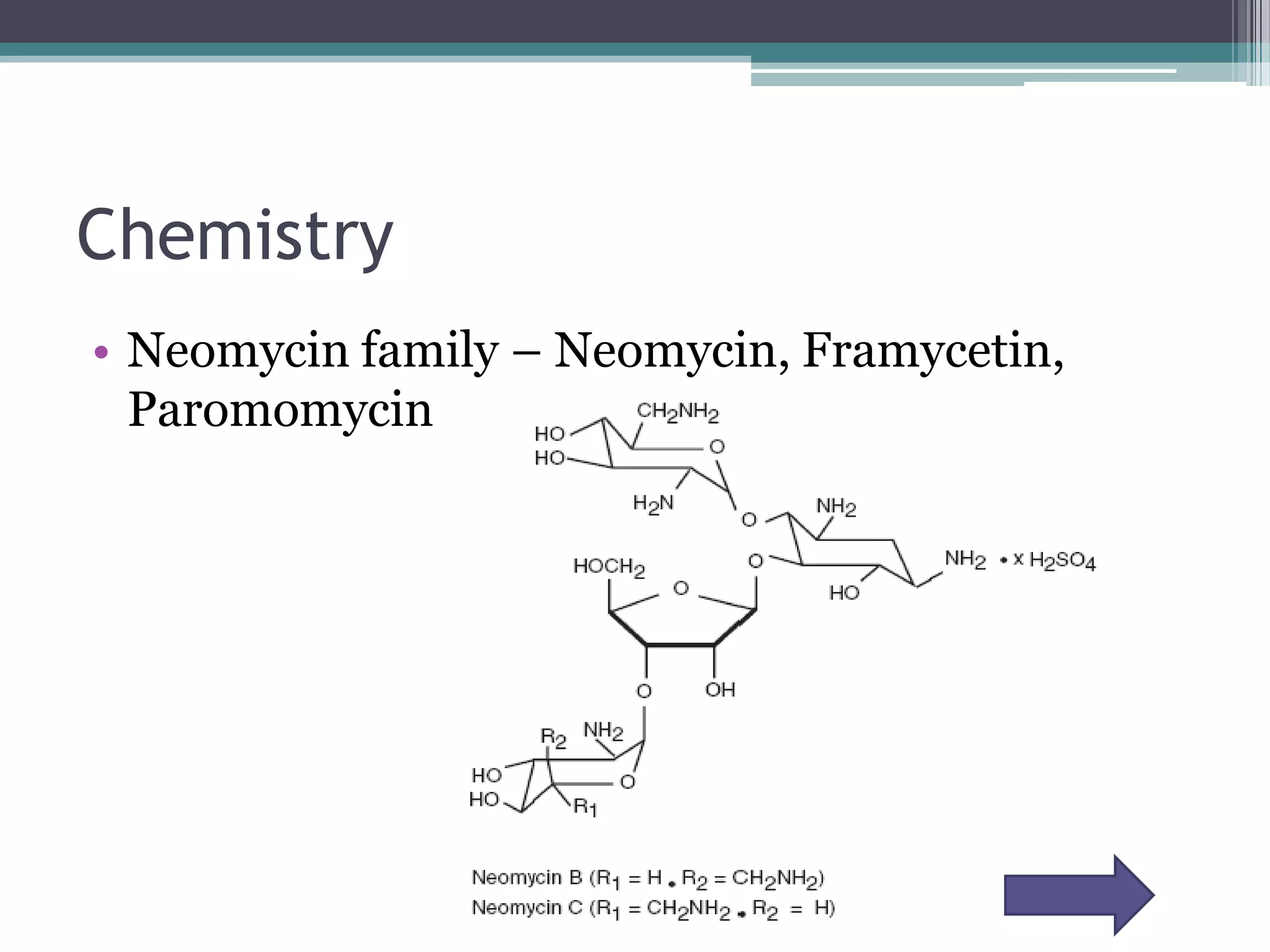
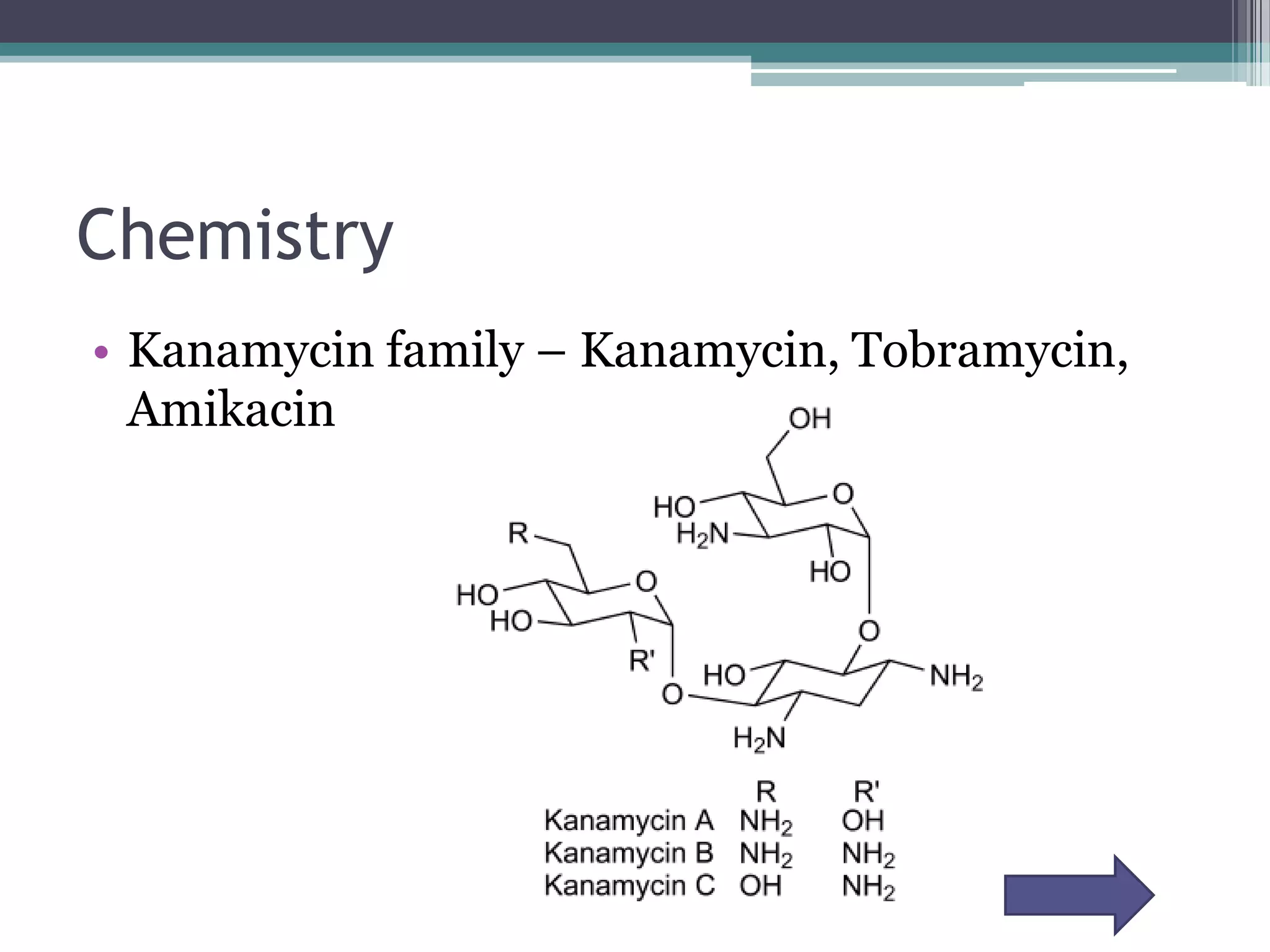
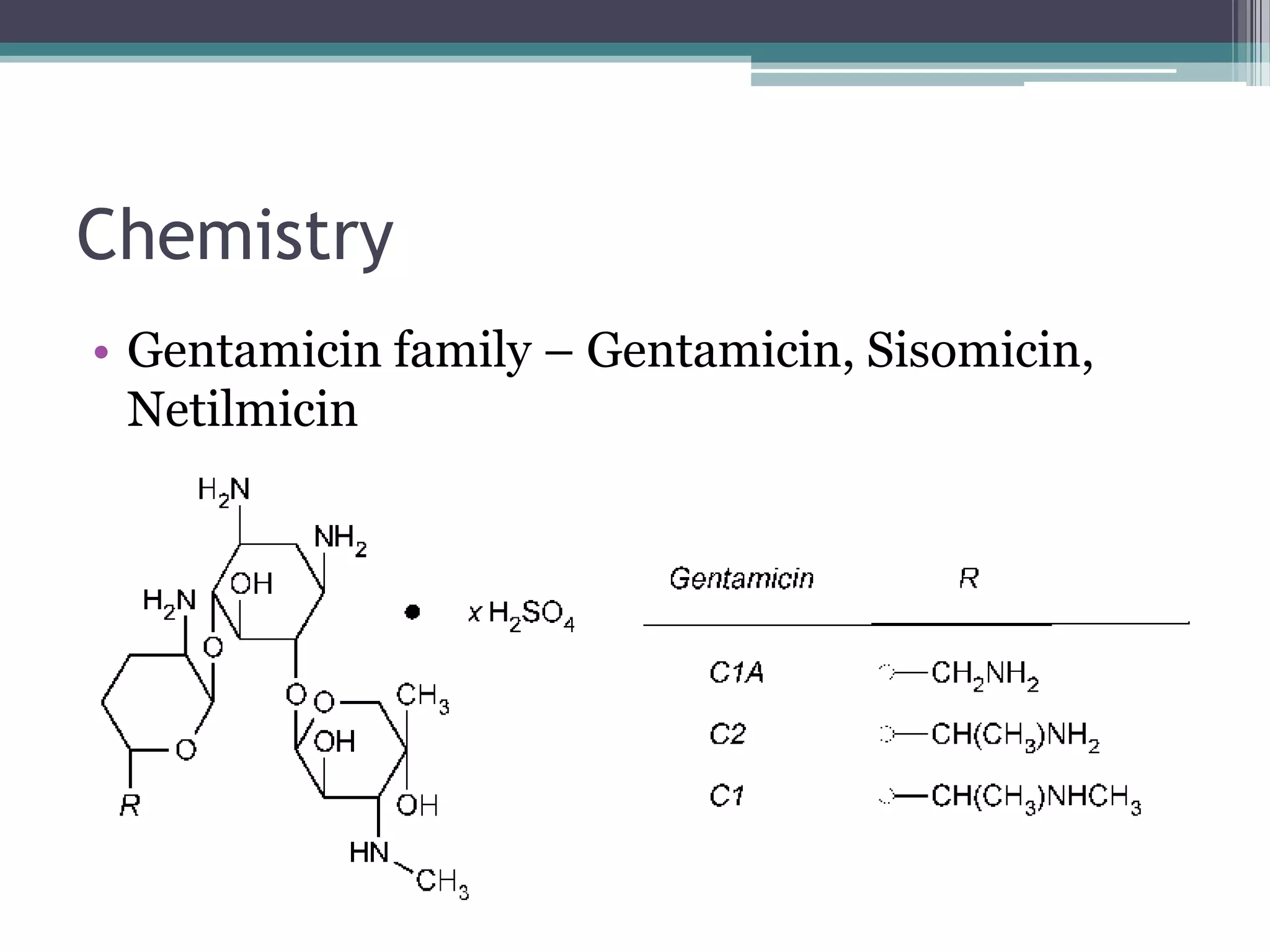
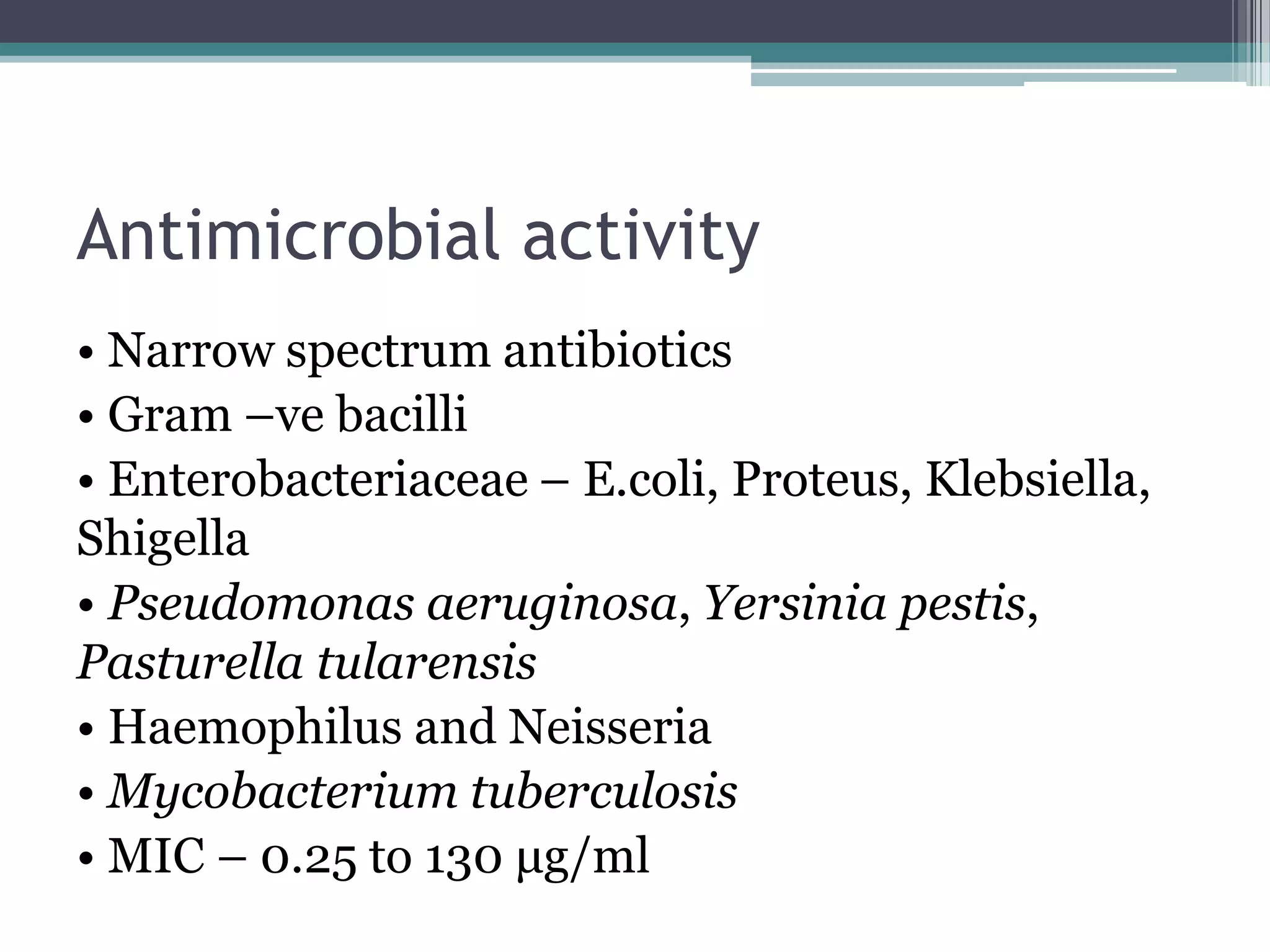
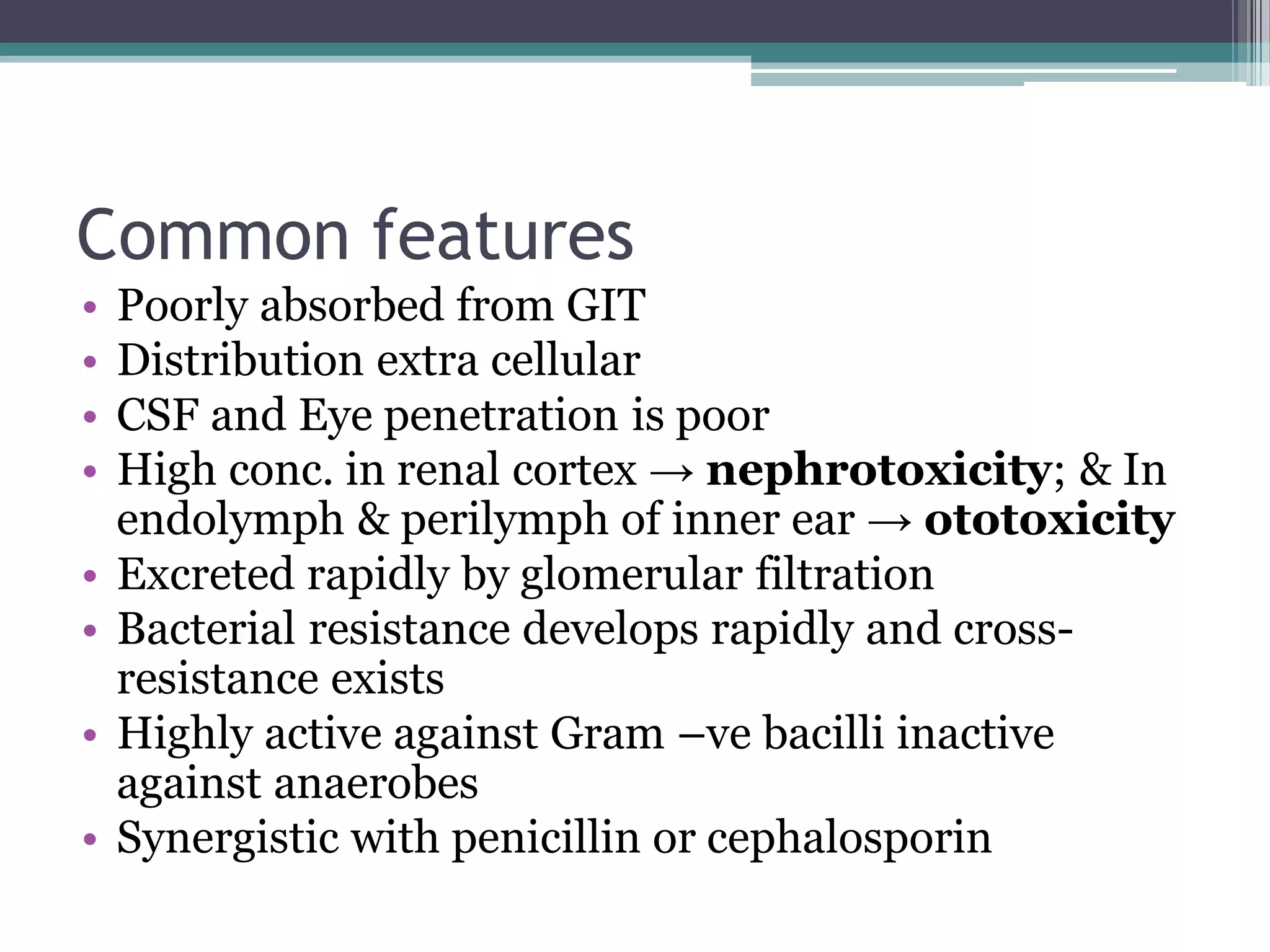
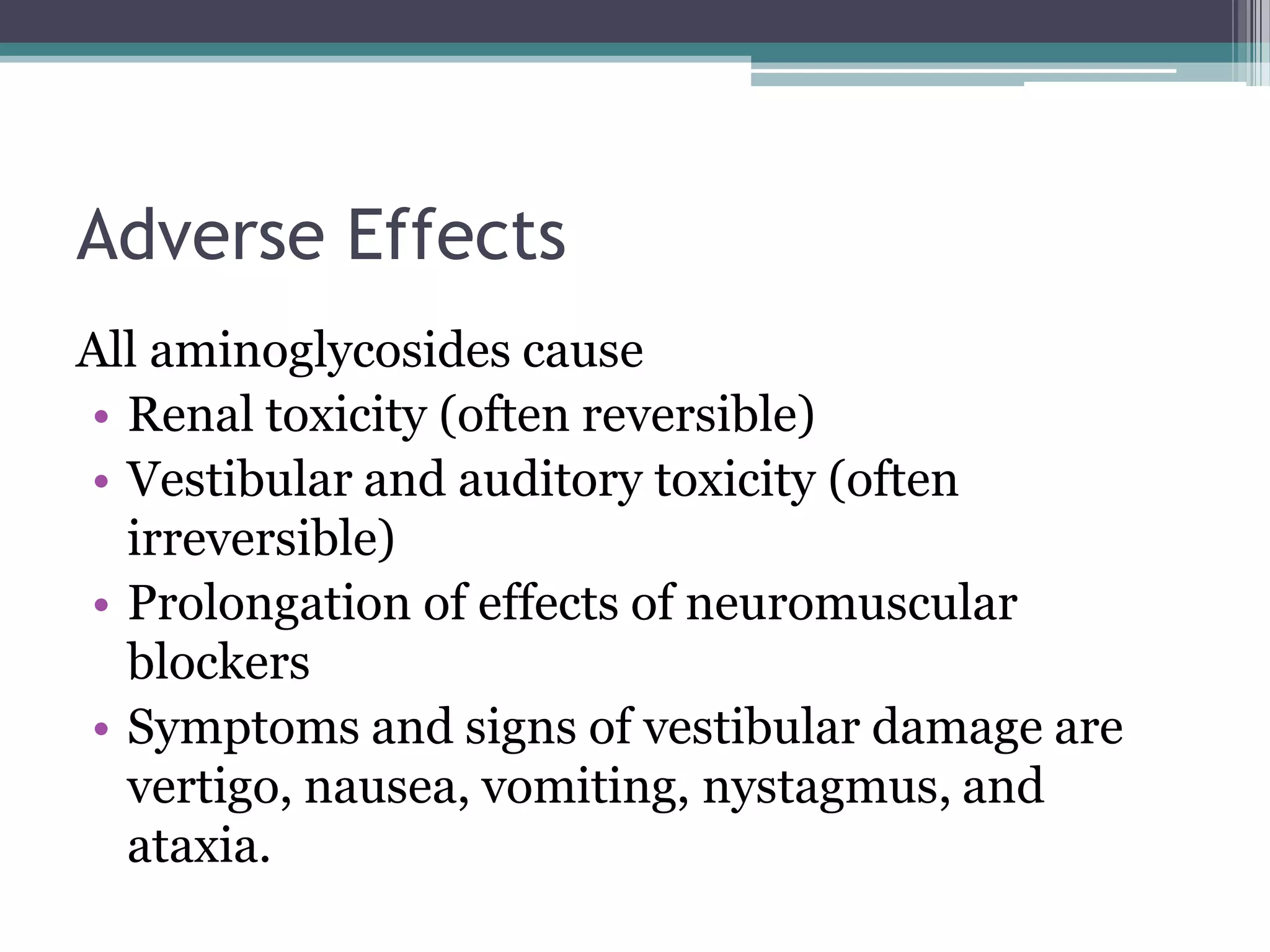
Aminoglycosides are a class of antibiotics that were first discovered in 1944 and are produced by actinomycetes bacteria. They work by interfering with bacterial protein synthesis and are bactericidal. They are narrow spectrum and primarily effective against gram-negative bacteria. Common examples include streptomycin, neomycin, kanamycin, and gentamicin. While effective antibiotics, aminoglycosides have the drawbacks of nephrotoxicity, ototoxicity, and rapid development of bacterial resistance. Their use requires monitoring of dosages and risks.















![References
• ^ MeSH Aminoglycosides ^ Massachusetts Institute of Technology (February 26, 2008).
"Bacterial 'battle for survival' leads to new antibiotic". Press release.
http://web.mit.edu/newsoffice/2008/antibiotics-0226.html. Retrieved December 1, 2010. ^ a b
Pharmamotion --> Protein synthesis inhibitors: aminoglycosides mechanism of action animation.
Classification of agents Posted by Flavio Guzmán on 12/08/08[self-published source?] ^ Shakil, Shazi; Khan, Rosina;
Zarrilli, Raffaele; Khan, Asad U. (2007). "Aminoglycosides versus bacteria – a description of the action,
resistance mechanism, and nosocomial battleground". Journal of Biomedical Science 15 (1): 5–14.
doi:10.1007/s11373-007-9194-y. PMID 17657587. ^ Levison, Matthew E. (July 2009). "Aminoglycosides:
Bacteria and Antibacterial Drugs". Merck Manual Professional.
http://www.merck.com/mmpe/sec14/ch170/ch170b.html. ^ "Aminoglycosides".
http://www.aic.cuhk.edu.hk/web8/aminoglycosides.htm. ^ Champney, W. S. (2001). "Bacterial Ribosomal
Subunit Synthesis A Novel Antibiotic Target". Current Drug Targets - Infectious Disorders 1 (1): 19–36.
doi:10.2174/1568005013343281. PMID 12455231. ^ Lorian, Victor (1996). Antibiotics in Laboratory
Medicine. Williams & Wilkins Press. pp. 589–90. ISBN 0-683-05169-5. ^ Feero, W. Gregory; Guttmacher,
Alan E.; Dietz, Harry C. (2010). "New Therapeutic Approaches to Mendelian Disorders". New England
Journal of Medicine 363 (9): 852–63. doi:10.1056/NEJMra0907180. PMID 20818846. ^ Wilschanski,
Michael; Yahav, Yaacov; Yaacov, Yasmin; Blau, Hannah; Bentur, Lea; Rivlin, Joseph; Aviram, Micha; Bdolah-
Abram, Tali et al. (2003). "Gentamicin-Induced Correction of CFTR Function in Patients with Cystic Fibrosis
andCFTRStop Mutations". New England Journal of Medicine 349 (15): 1433–41.
doi:10.1056/NEJMoa022170. PMID 14534336. ^ Falagas, Matthew E; Grammatikos, Alexandros P;
Michalopoulos, Argyris (2008). "Potential of old-generation antibiotics to address current need for new
antibiotics". Expert Review of Anti-infective Therapy 6 (5): 593–600. doi:10.1586/14787210.6.5.593.
PMID 18847400. ^ Durante-Mangoni, Emanuele; Grammatikos, Alexandros; Utili, Riccardo; Falagas,
Matthew E. (2009). "Do we still need the aminoglycosides?". International Journal of Antimicrobial Agents
33 (3): 201–5. doi:10.1016/j.ijantimicag.2008.09.001. PMID 18976888.](https://image.slidesharecdn.com/aminoglycoside-13145406200662-phpapp01-110828091127-phpapp01/75/Aminoglycoside-23-2048.jpg)