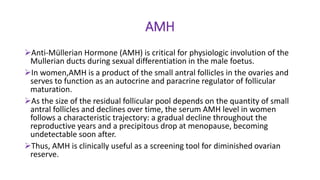
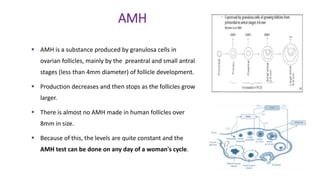
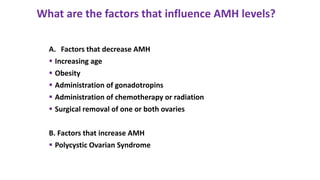
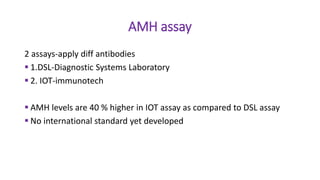
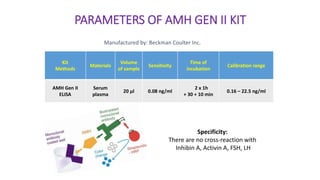
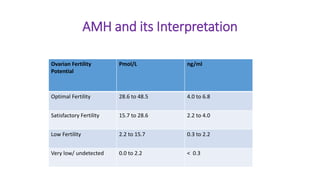
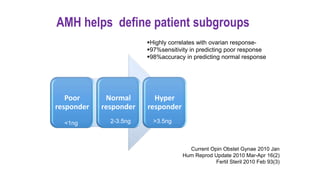
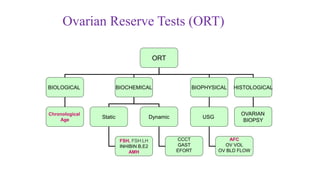
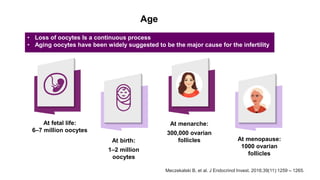
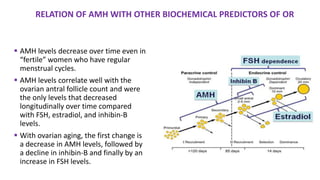
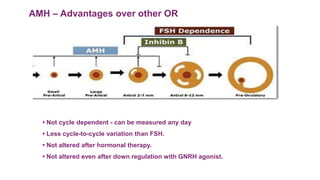
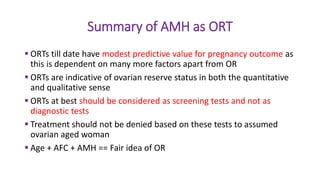
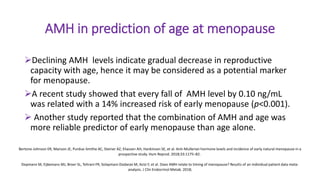
The document discusses the role of anti-Müllerian hormone (AMH) in fertility, emphasizing its importance as a biomarker for ovarian reserve and its clinical applications in assisted reproductive technology, fertility preservation, and various gynecological conditions like polycystic ovarian syndrome (PCOS). It details how AMH levels can aid in individualizing treatment plans for women, particularly those at risk of diminished ovarian function or undergoing cancer treatments, while also discussing its limitations as a sole predictor of pregnancy outcomes. Furthermore, the document outlines the implications of AMH testing in pediatric reproductive endocrinology and its potential use as a diagnostic marker in both males and females.