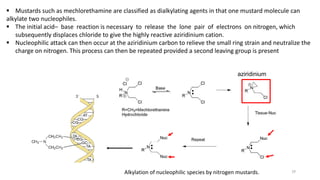
This document discusses alkylating agents, specifically nitrogen mustards, as a prominent class of anticancer drugs, detailing their historical background, mechanisms of action, and applications in treating various cancers. It highlights the biochemical processes involved in DNA alkylation, the development of different nitrogen mustard compounds, and their effectiveness against solid tumors and hematological malignancies. Additionally, it addresses the common side effects and the pharmacological strategies to enhance drug selectivity and reduce toxicity.










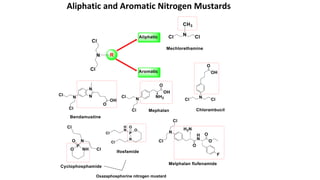









![➢Melphalan
▪ Melphalan chemically is 4-[bis(2-chloroethyl)amino]-L-phenylalanine and it is a phenylalanine
derivative of nitrogen mustard that acts as a bifunctional alkylating agent.
▪ Because L-phenylalanine is a precursor to melanin, it was thought that L-phenylalanine
nitrogen mustard might accumulate in melanomas.
▪ Used in multiple myeloma, ovarian cancer and Malignant melanoma
▪ Suitable for oral administration and Iv infusion](https://image.slidesharecdn.com/alkylatingagents-nitrogenmustardspartone2024-240517144654-6f8e1261/85/Nitrogen-Mustards-Medicinal-Chemistry-College-of-Pharmacy-22-320.jpg)













