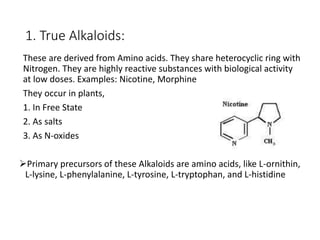
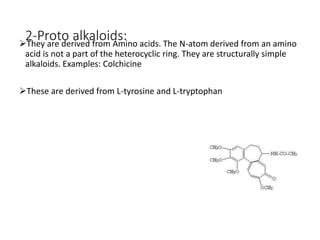
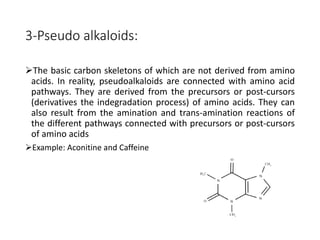
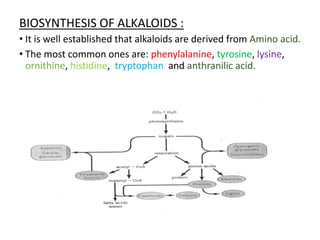
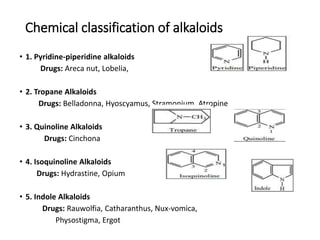
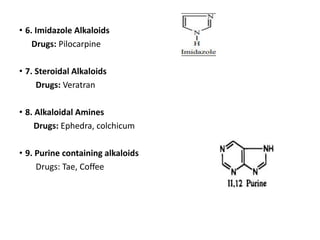
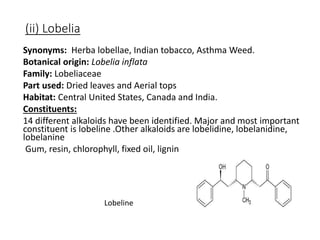
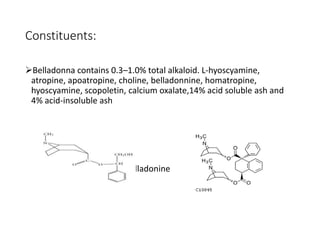
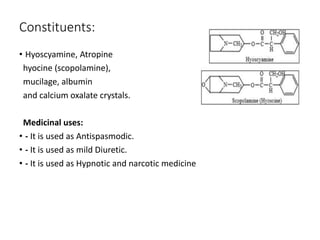
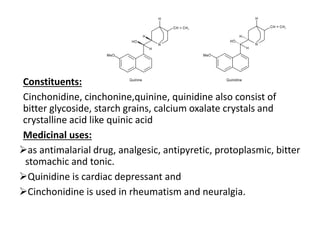
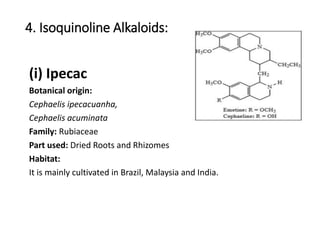
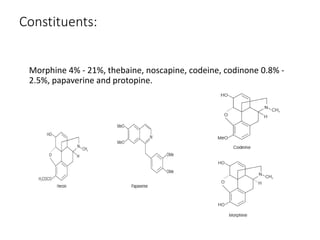
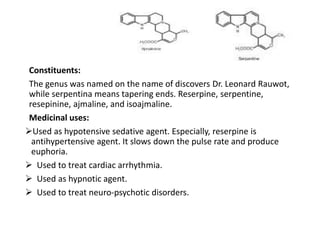
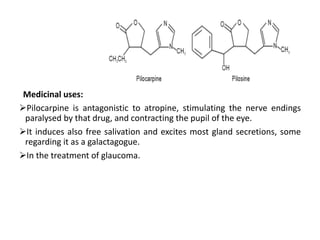
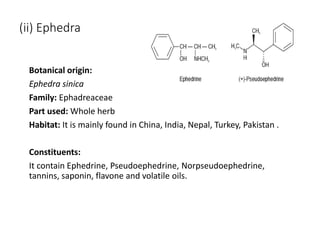
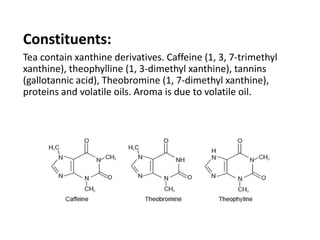
This document provides an overview of alkaloids. It defines alkaloids as nitrogenous organic compounds that are typically bitter and have pharmacological effects. Alkaloids are found in various plants, animals, and fungi. The document discusses the distribution, extraction, classification, and examples of important alkaloids like morphine, codeine, caffeine, and quinine. It also summarizes key information about specific alkaloid-containing plants such as opium, belladonna, coca, and ipecac.