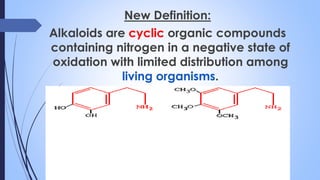
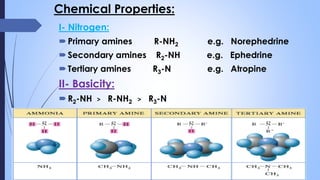
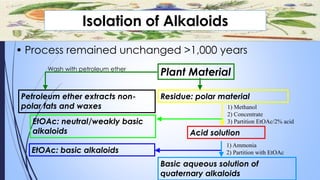
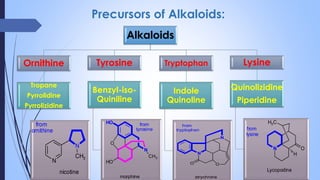
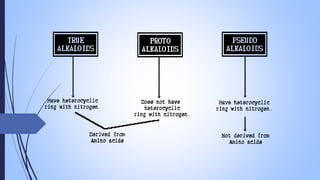
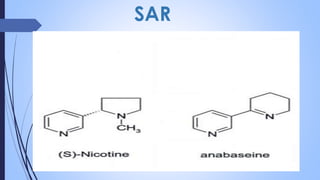
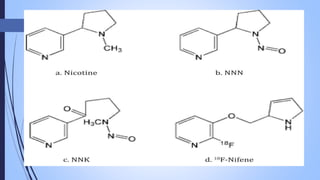
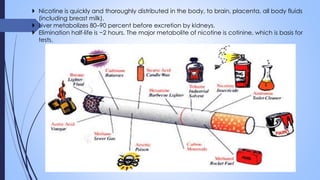
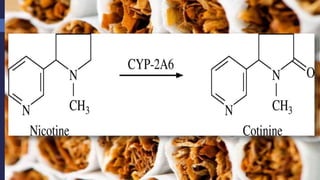
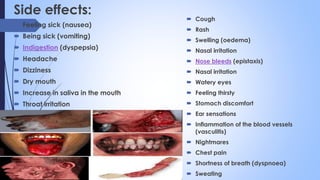
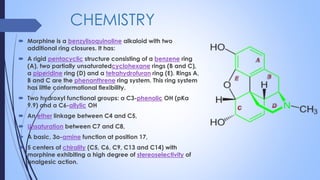
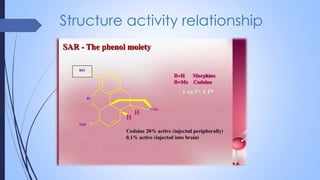
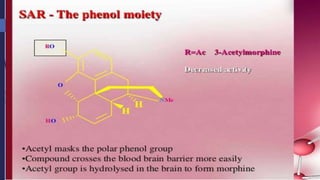
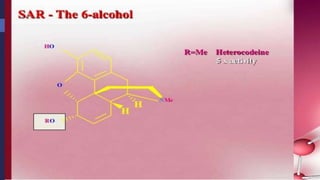
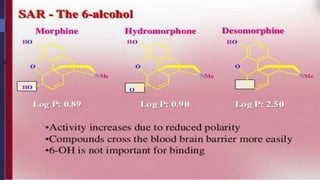
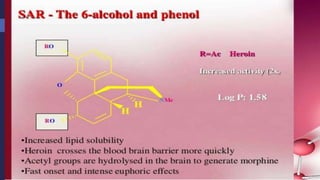
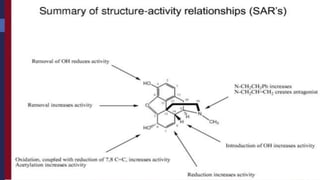
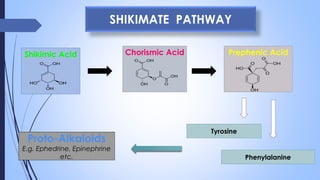
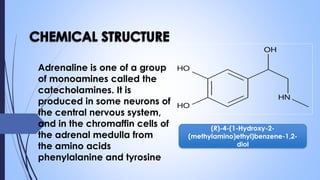
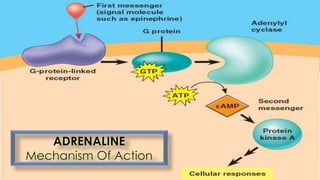
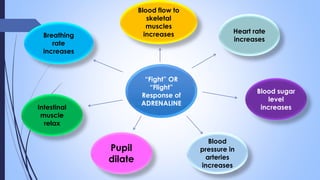
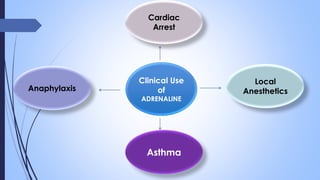
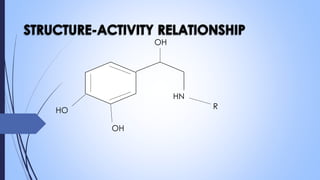
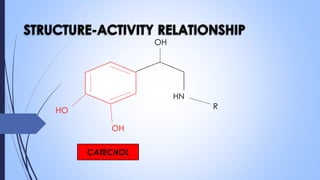
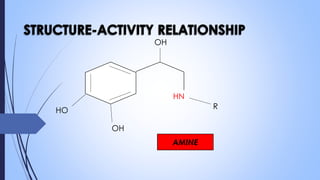
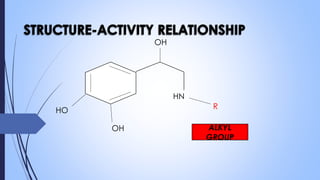
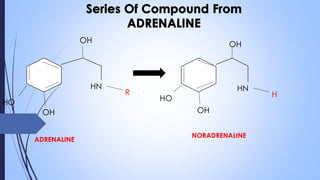
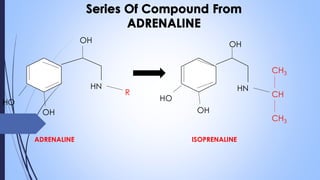
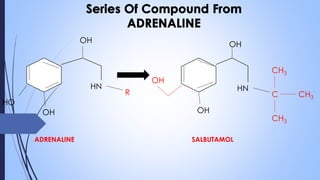
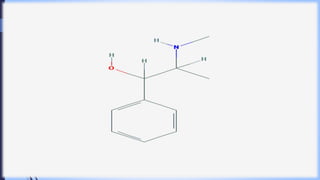
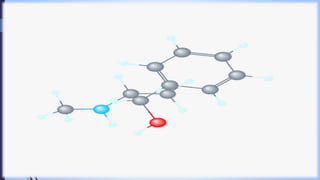
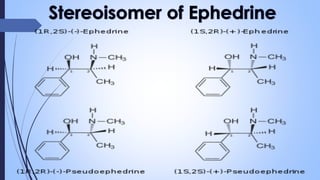
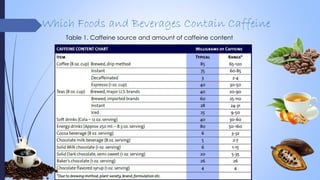
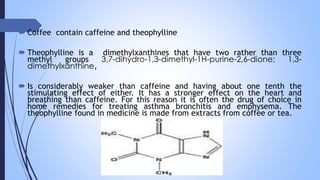
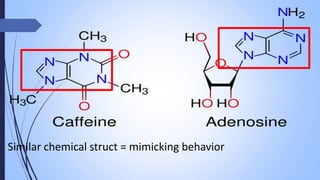
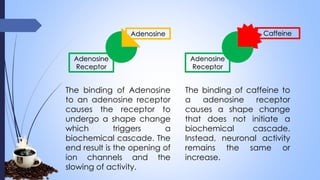
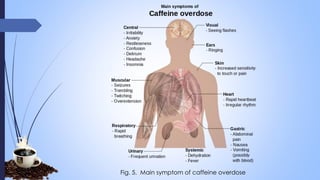
This document provides a comprehensive overview of alkaloids, detailing their definitions, history, classification, and effects on humans and plants. It includes information on various specific alkaloids like nicotine and morphine, their chemical structures, uses, and potential side effects. The text also touches on the biosynthesis and methods for isolation and purification of alkaloids.