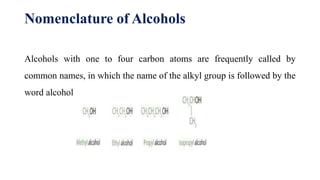
This document provides information about alcohols including their functional group, types, nomenclature, physical properties, and chemical properties. It defines alcohols as organic compounds that contain a hydroxyl functional group and can be made through fermentation. The document outlines the rules for naming alcohols based on carbon chain length and functional groups. It also describes some of the key physical properties of alcohols such as being colorless and flammable liquids, and their chemical properties including oxidation, combustion, and dehydration reactions.