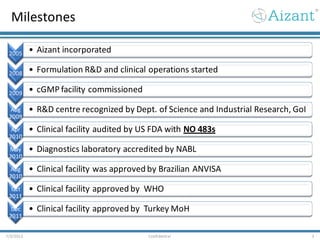
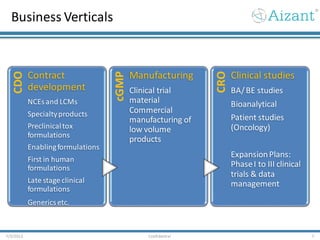
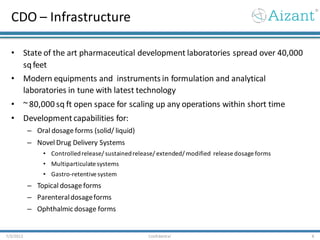
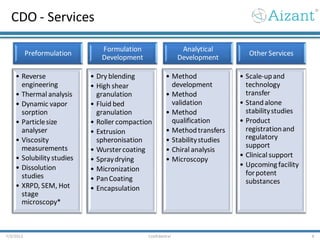
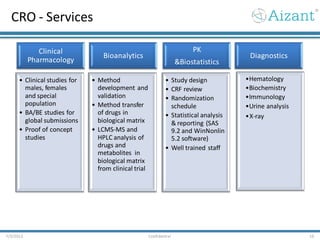
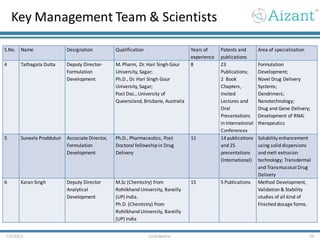
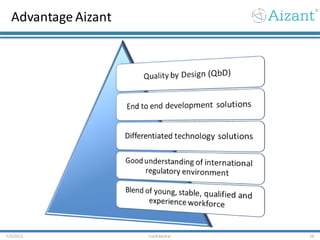
Aizant Drug Research Solutions provides integrated drug development solutions including contract development organization (CDO) and contract research organization (CRO) capabilities. The presentation outlines Aizant's business verticals, infrastructure, services, certifications, management team, experience, expansion plans, and advantages. It also provides contact information for Aizant.