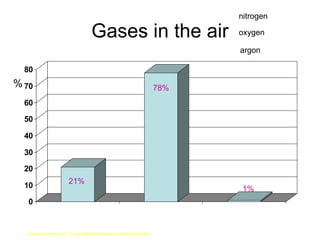
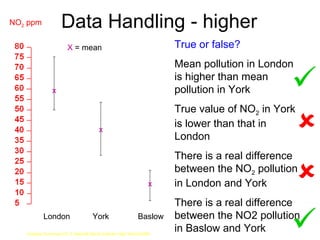
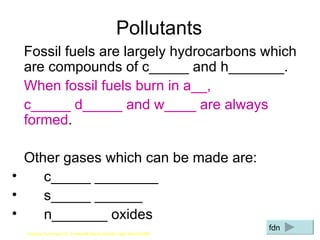
The document discusses air quality and pollution. It defines key terms like air quality, combustion, photosynthesis, pollution, and respiration. It provides data on the composition of gases in air. It also discusses pollutants like nitrogen oxides, sulfur dioxide, carbon monoxide, and carbon dioxide that are produced by combustion of fossil fuels. The harmful effects of these pollutants are outlined. Methods to reduce pollution from power stations, transportation, and taxation are also mentioned.