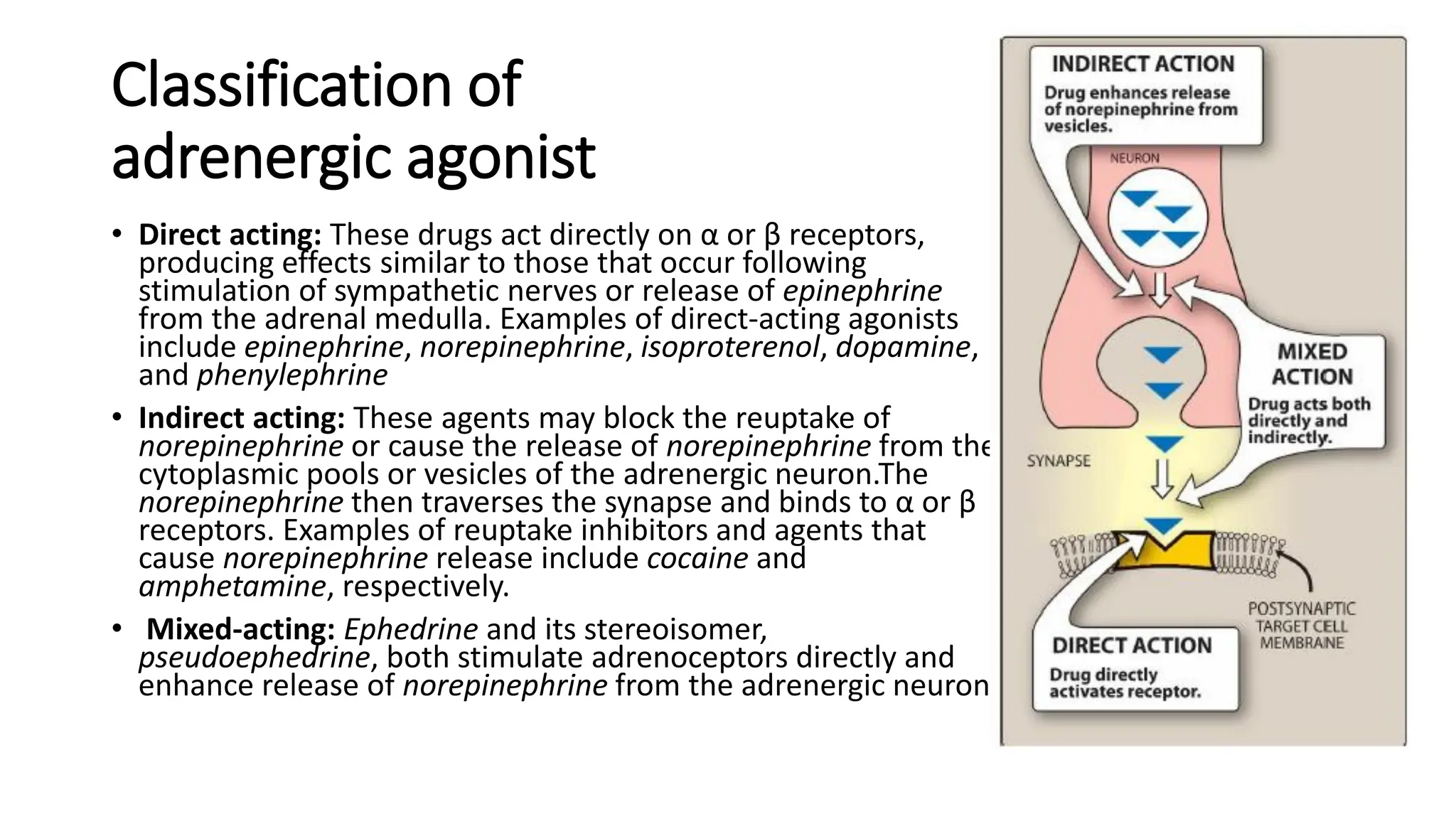
Adrenergic agonists can be direct-acting or indirect-acting. Direct-acting agonists like epinephrine directly stimulate alpha and beta receptors. Indirect agonists like cocaine enhance the effects of norepinephrine by blocking its reuptake from synapses. Adrenergic receptors are divided into alpha and beta types with multiple subtypes that influence the affinity of agonists. Activation of different receptor subtypes mediates various physiological effects including changes in cardiovascular function, bronchodilation, lipolysis, and more.







![α2 Receptors
• These receptors are located primarily on sympathetic presynaptic nerve endings and control the
release of norepinephrine.
• When a sympathetic adrenergic nerve is stimulated, a portion of the released norepinephrine
“circles back” and reacts with α2 receptors on the presynaptic membrane.
• Stimulation of α2 receptors causes feedback inhibition and inhibits further release of
norepinephrine from the stimulated adrenergic neuron.
• This inhibitory action serves as a local mechanism for modulating norepinephrine output when
there is high sympathetic activity. [Note: In this instance, by inhibiting further output of
norepinephrine from the adrenergic neuron, these receptors are acting as inhibitory
autoreceptors]
• α2 Receptors are also found on presynaptic parasympathetic neurons. Norepinephrine released
from a presynaptic sympathetic neuron can diffuse to and interact with these receptors, inhibiting
acetylcholine release.
• This is another mechanism to modulate autonomic activity in a given area. In contrast to α1
receptors, the effects of binding at α2 receptors are mediated by inhibition of adenylyl cyclase
and by a fall in the levels of intracellular cAMP.](https://image.slidesharecdn.com/adrenergicagonists-231216080849-20d7ff0a/75/adrenergic-agonists-for-all-medical-students-8-2048.jpg)




















![Amphetamine
• The marked central stimulatory action of amphetamine [am-FET-a-
meen] is often mistaken by drug abusers as its only action.
• However, the drug can also increase blood pressure significantly by α1
agonist action on the vasculature, as well as β1 stimulatory effects on
the heart. Its actions are mediated primarily through an increase in
nonvesicular release of catecholamines such as dopamine and
norepinephrine from nerve terminals.
• Thus, amphetamine is an indirect-acting adrenergic drug. The actions
and therapeutic uses of amphetamine and its derivatives are
discussed with CNS stimulants](https://image.slidesharecdn.com/adrenergicagonists-231216080849-20d7ff0a/75/adrenergic-agonists-for-all-medical-students-30-2048.jpg)


