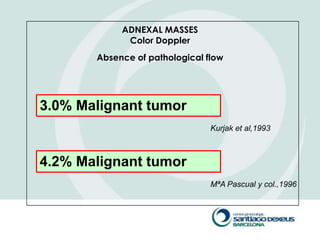
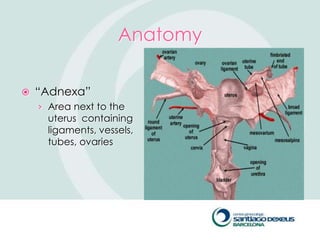
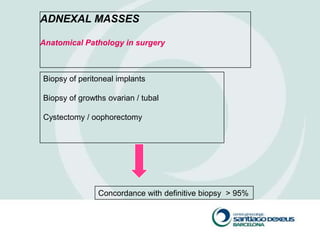
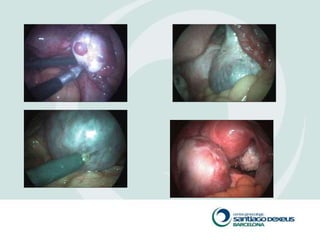
1) Adnexal masses are common gynecological findings that are usually benign. Differential diagnosis includes physiologic cysts, endometriomas, fibroids, and malignancies.
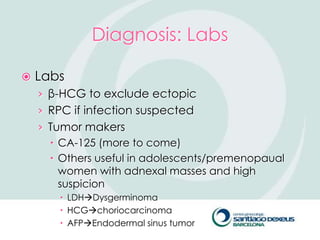
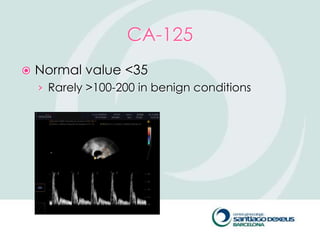
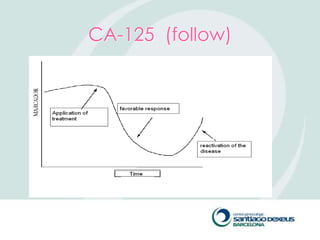
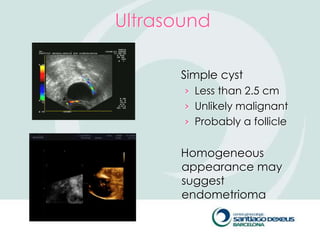
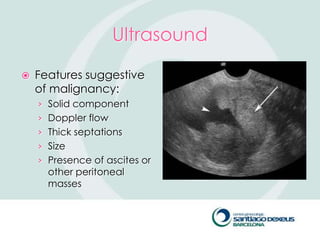
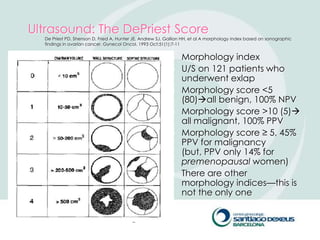
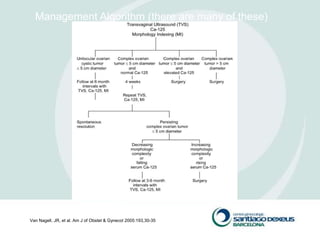
2) Evaluation involves history, physical exam, ultrasound, and tumor markers like CA-125. Ultrasound can identify features suggestive of malignancy such as solid components, blood flow, thick septations, large size, and ascites.
3) For premenopausal women, small unilateral cystic masses may be followed with ultrasound in a few months. Larger or complex masses require surgery. Postmenopausal women with cysts under 10cm may also be followed, but require close monitoring for changes that