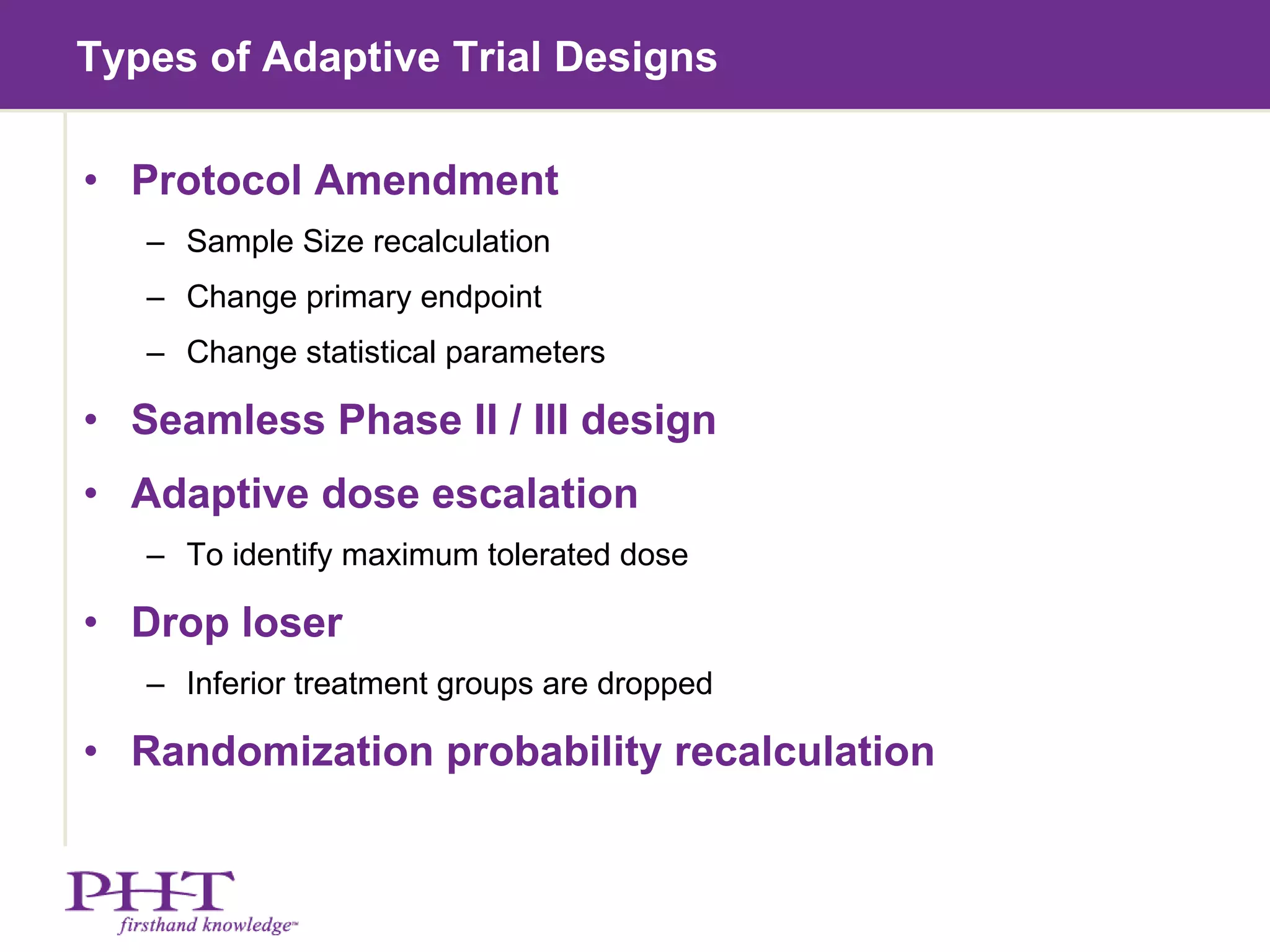
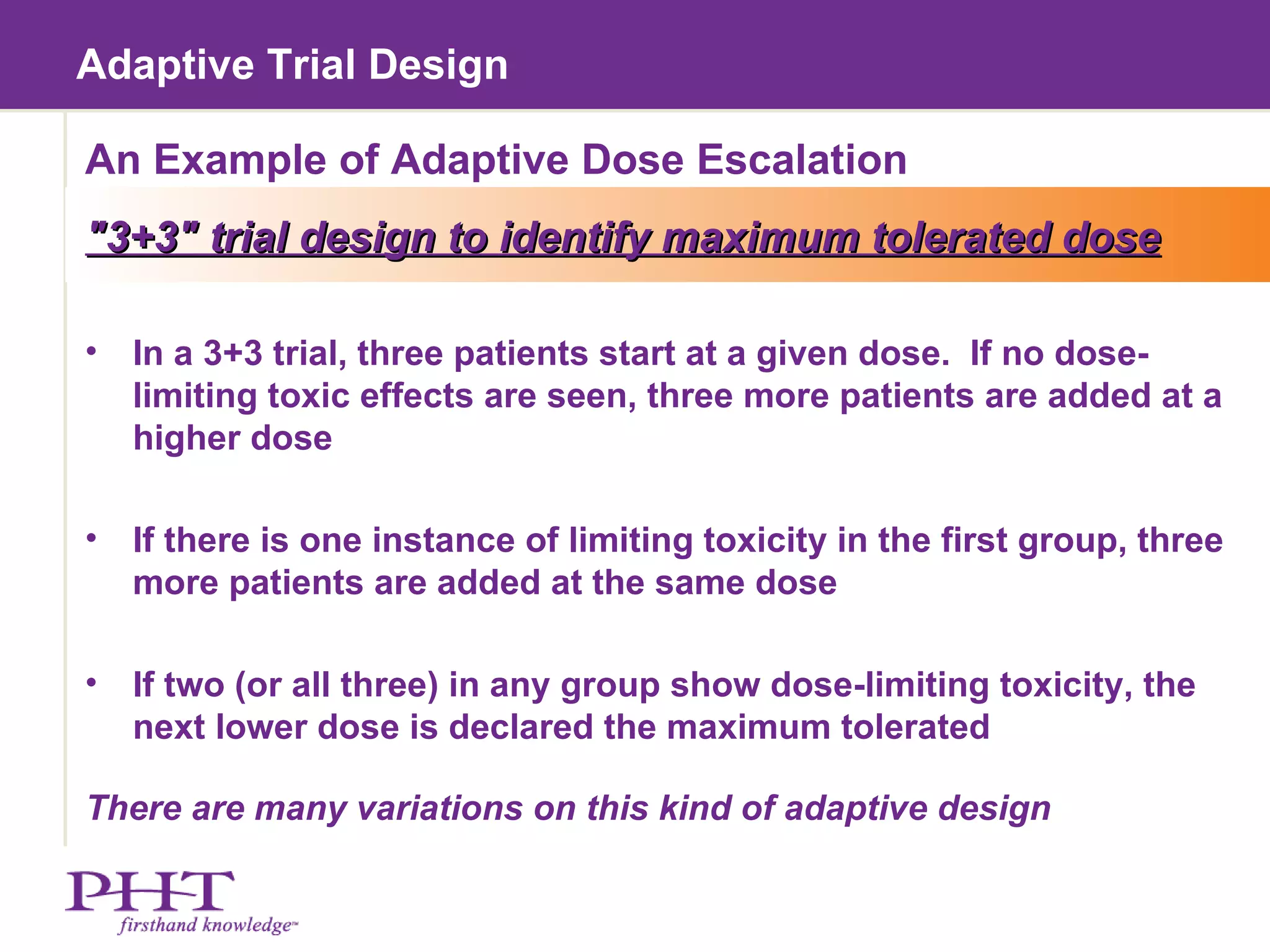
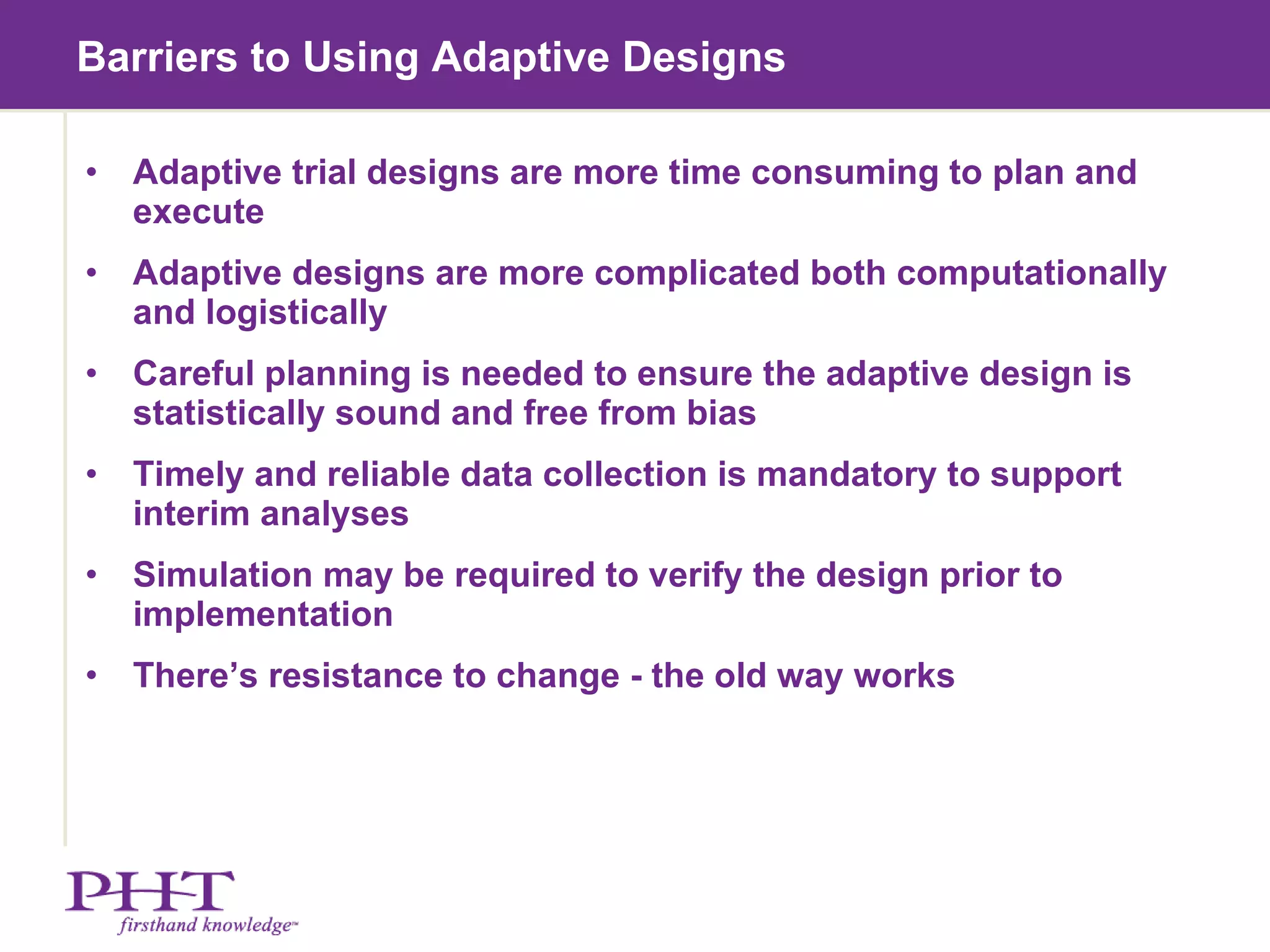
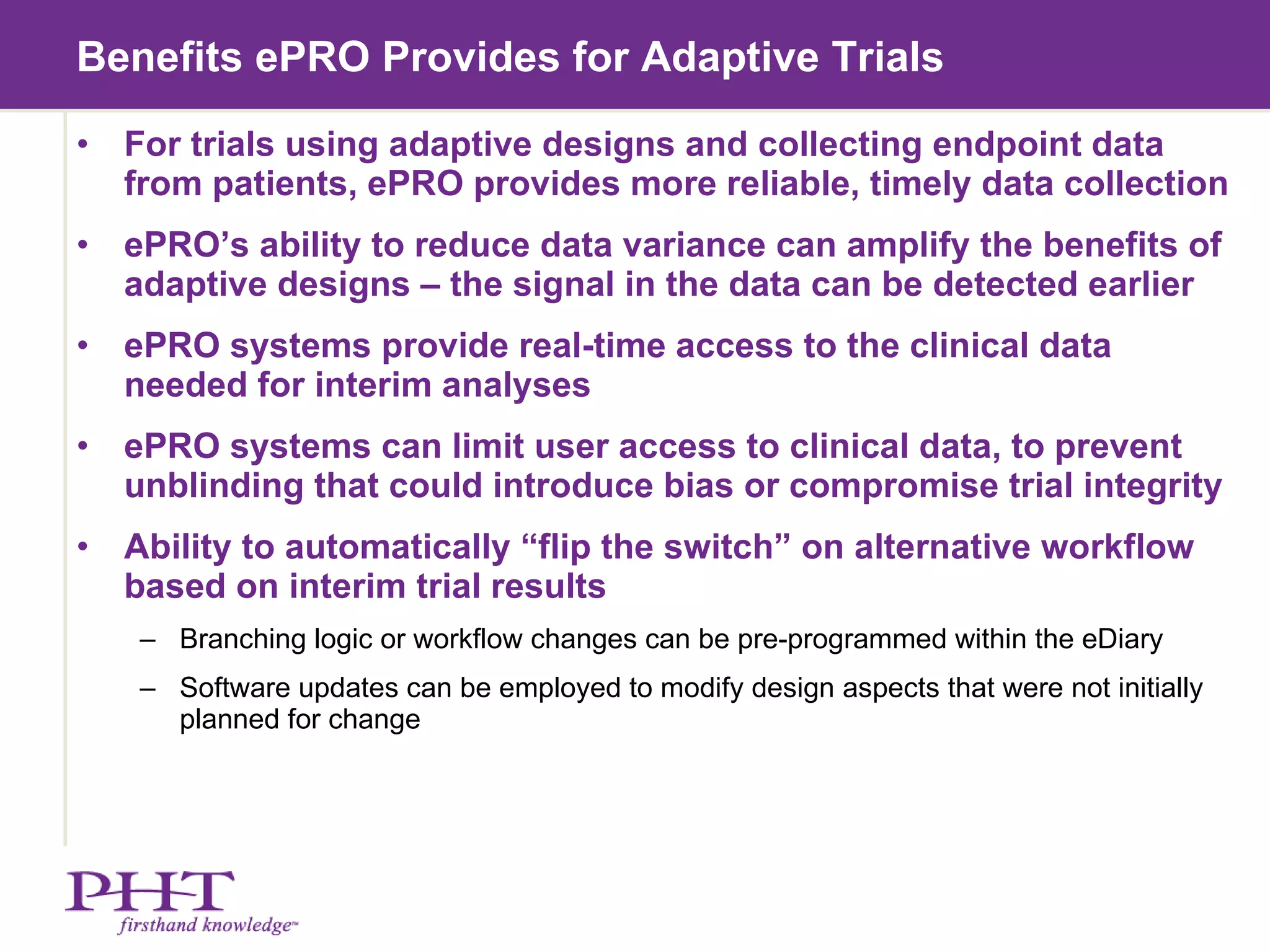
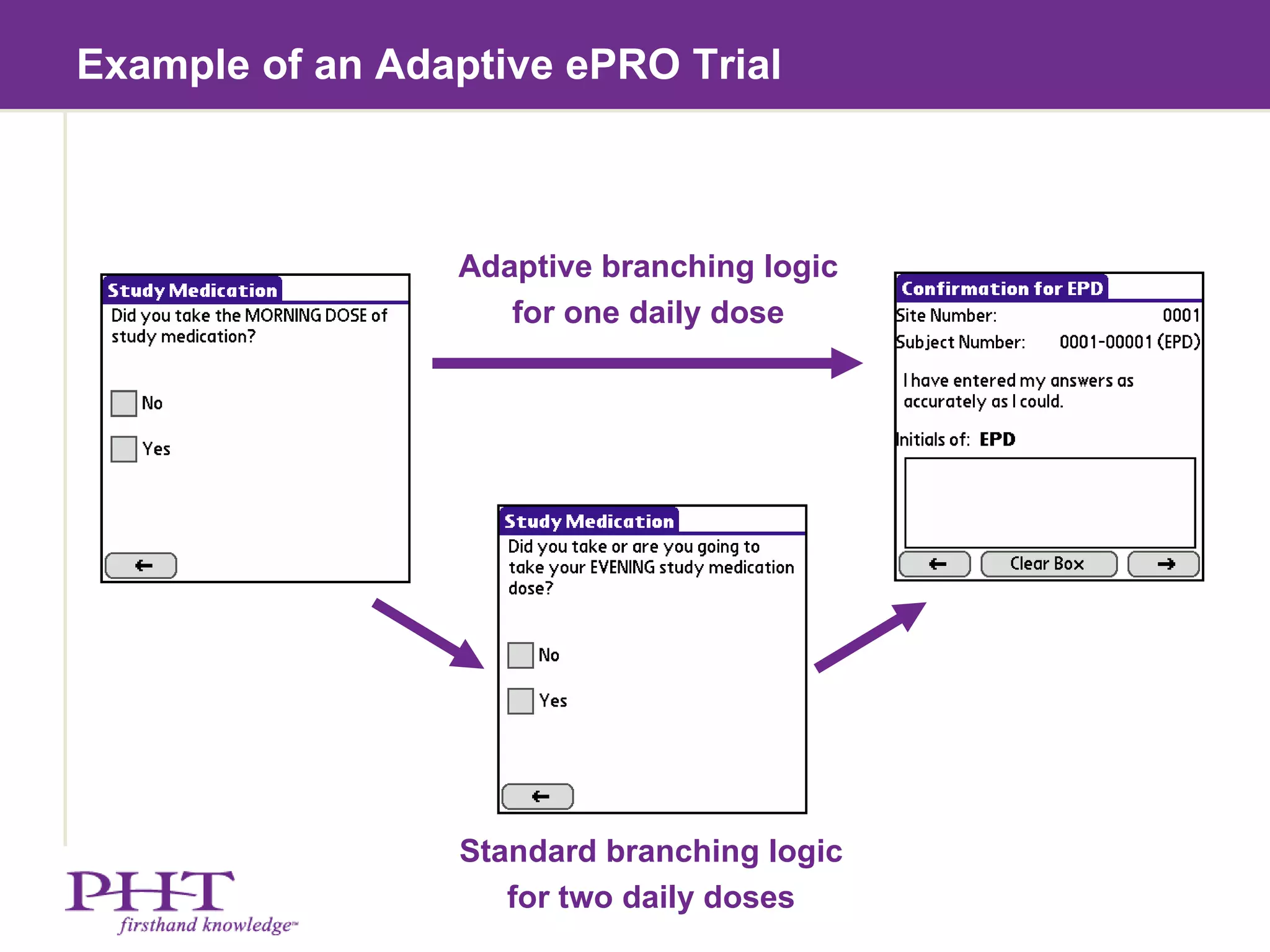
The document discusses using electronic patient-reported outcomes (ePRO) in adaptive clinical trial designs. It defines adaptive trials as designs that can flexibly change aspects of the trial based on interim analyses of accumulating data. Examples of adaptive changes include modifying the sample size, endpoints, randomization probabilities, or treatment groups. ePRO provides reliable, timely data collection to support interim analyses in adaptive trials. It also allows automatic changes to data collection workflows or questionnaires based on adaptive trial decisions.
![Using ePRO in Adaptive Trial Design CBI 4 th PRO Conference Dwight Cooper Global Director, Technical Services PHT Corporation (617) 973-1838 [email_address] 28 May 2009](https://image.slidesharecdn.com/cbieproadaptivedesign-may2009final-090608155950-phpapp01/75/ePro-Adaptive-Design-1-2048.jpg)