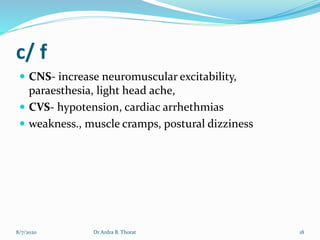
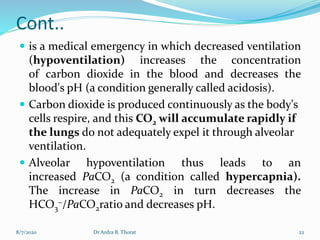
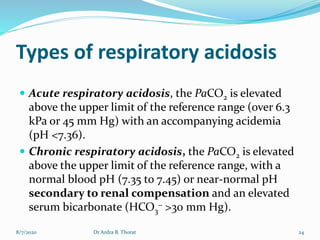
The document provides an overview of acid-base balance in the body, emphasizing the regulation of hydrogen ion concentration for maintaining homeostasis and normal pH levels, crucial for enzymatic reactions. It discusses the concepts of acidosis and alkalosis, their causes, symptoms, and diagnostic tests, along with treatment strategies for related conditions. Various types of metabolic and respiratory imbalances are detailed, highlighting their physiological implications and potential complications.

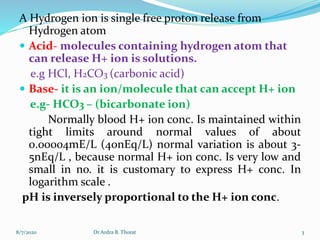
![1
pH = log -------- = - log [H+]
[H+]
e.g normal H + ion conc. Is 40nEq/L
(0.00000004 Eq/L) :. Normal pH is
pH= -log[0.00000004]
pH= 7.4
8/7/2020 4Dr Ardra B. Thorat](https://image.slidesharecdn.com/acid-base-200807105819/85/Acid-base-4-320.jpg)