Embed presentation
Download to read offline

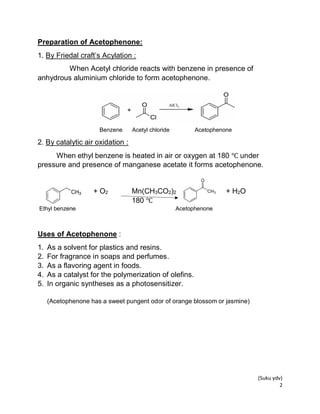
Acetophenone is an organic compound that is the simplest aromatic ketone. It has the formula C6H5C(O)CH3 and is a methyl ketone where one methyl group of acetone is replaced by a phenyl group. Acetophenone is a colorless, viscous liquid with a sweet pungent odor. It can be prepared through Friedal-Crafts acylation using acetyl chloride and benzene or through catalytic air oxidation of ethylbenzene. Acetophenone is used as a solvent, in fragrances, as a flavoring agent, and in organic syntheses.

