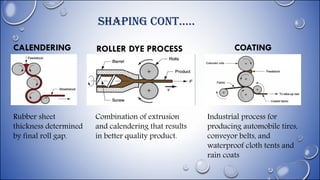
The document provides an overview of synthetic rubber manufacturing, including its classification, processing, and comparisons with natural rubber. It details the processes involved, such as mastication, mixing, shaping, and vulcanization, highlighting the importance of various additives and techniques. Additionally, it traces the history of synthetic rubber since its creation in 1909 and explains the benefits of vulcanization in enhancing rubber properties.