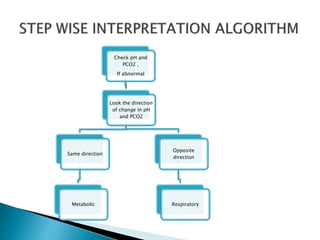
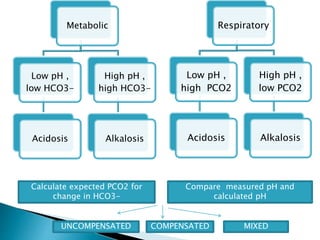
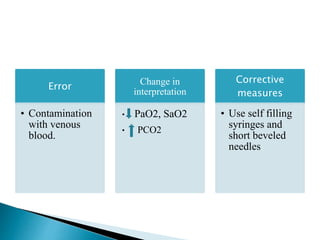
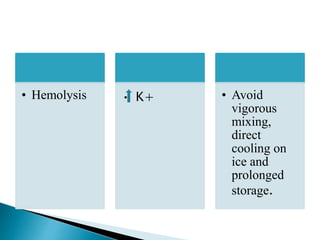
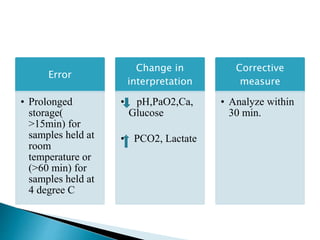
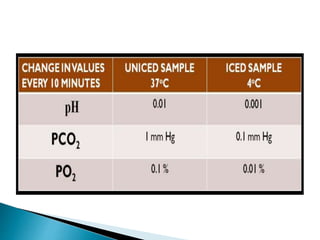
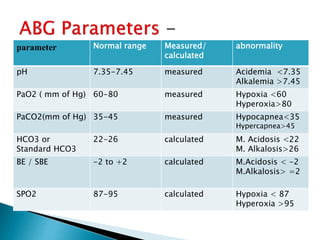
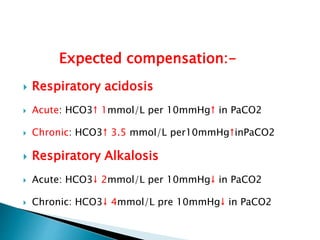
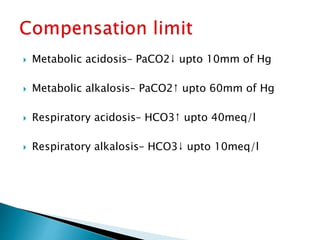
This document provides an overview of arterial blood gas (ABG) analysis and interpretation. It discusses indications for ABG testing, appropriate sampling sites, and precautions. Key parameters measured in an ABG such as pH, PaCO2, PaO2, HCO3, and SaO2 are defined. Methods for interpreting ABG results, including evaluating for respiratory vs. metabolic causes and compensation, are outlined. Common errors in sampling technique and their effects are reviewed.









![pH = -log(H+) =log(1/H+)
At a pH of 7.4 H+ ion conc = 40 mmol/L
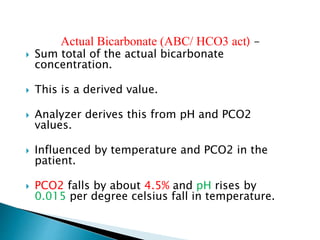
Henderson–Hasselbach equation:-
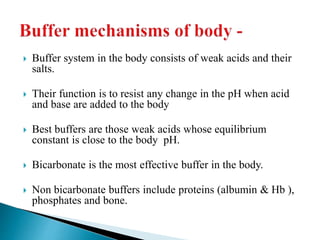
The bicarbonate buffer system is routinely monitored clinically.
CO2 +H2O ↔H+ +HCO3
−
The pK of this reaction is 6.1
pH = 6.1+ log[HCO3
− ]/[CO2]=
= 6.1+ log [24/40×0.03] = 6.1+ log [20 ] = 6.1+1.3= 7.4
pH of less than 6.8 or greater than 7.8 is considered incompatible with
life.](https://image.slidesharecdn.com/abginterpretation-keshav-191124140824/85/Abg-interpretation-keshav-21-320.jpg)
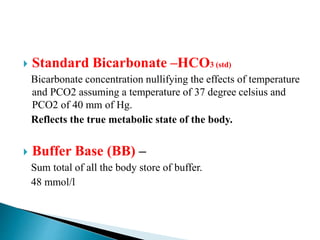
![ =base/ acid =log[HCO3
− ]/[CO2] = 24/0.03×40 = 20:1
Buffer ratio is 20 :1 for HCO3 / H2CO3 BUFFER.
as long as the buffer ratio is maintained the pH will
not change, even though the amount of hydrogen
changes.](https://image.slidesharecdn.com/abginterpretation-keshav-191124140824/85/Abg-interpretation-keshav-22-320.jpg)
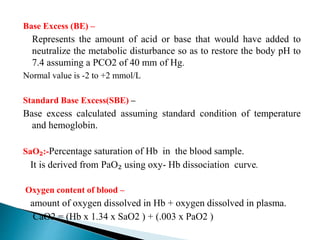





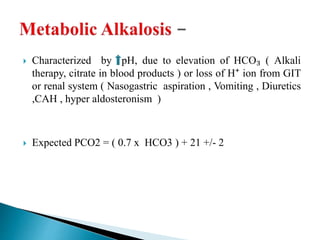
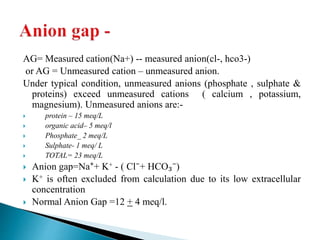
![ Metabolic acidosis
PCO2↓ 1.25mm Hg/1mmol/L↓ HCO3
Expected PCO₂ = [1.5xHCO3] + (8 ± 2)
(Winter’s equation)
Metabolic alkalosis
PCO2↑ 0.75mm Hg/1mmol/L↑ HCO3
Expected PCO2 = ( 0.7 x HCO3 ) +(21 +/- 2)](https://image.slidesharecdn.com/abginterpretation-keshav-191124140824/85/Abg-interpretation-keshav-32-320.jpg)
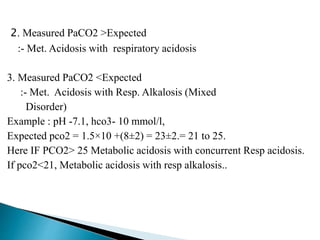
![ Characterized by pH , due to either HCO₃- or H+;
body tries to compensate by RR & washing out
CO₂.
Expected PCO₂ = [1.5xHCO3-] + 8±2
(Winters equation)
Interpretation of measured PaCO₂
1. Measured PaCO2 = Expected
:- Met. Acidosis with appropriate Compensation](https://image.slidesharecdn.com/abginterpretation-keshav-191124140824/85/Abg-interpretation-keshav-35-320.jpg)


![ Desired bicarbonate level is usually taken as 15 meq/L to tide
over critical situation.
Hco3 defict = [ Hco3( desired)- Hco3 act] × wt(kg) × 0.3
Half of the calculated dose is administered in 1-4 hr.
Further requirement will depend upon clinicalassessment and
repeat meaurement.
Objective is to maintain pH slightly above 7.2.
Inj sodabicarb may be given as bolus at dose of 1 meq/kg.
Injection sodabicarb (8.4%) - 1meq/ml.
osmolarity 2000mosm/L.](https://image.slidesharecdn.com/abginterpretation-keshav-191124140824/85/Abg-interpretation-keshav-42-320.jpg)