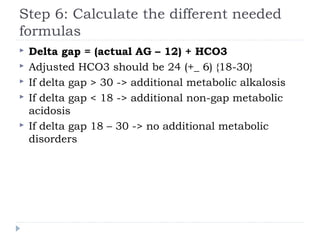
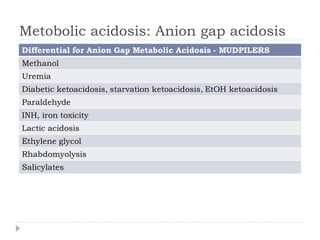
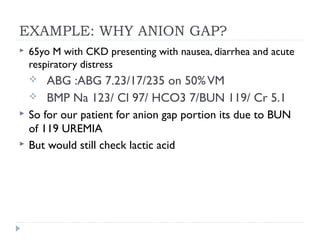
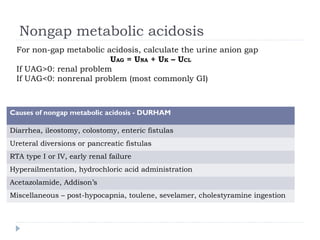
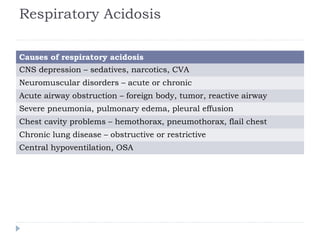
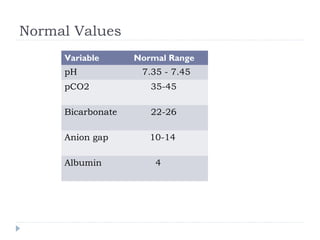
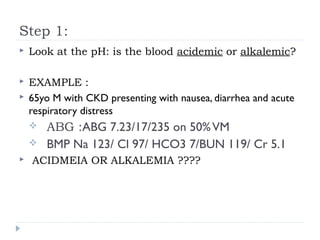
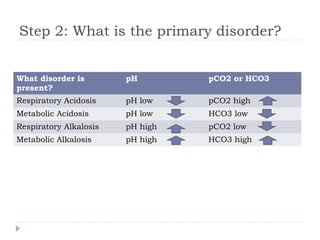
This document outlines the steps for analyzing arterial blood gases (ABGs) and acid-base disorders: 1) Determine if the pH is acidic or alkaline, 2) Identify the primary disorder (respiratory or metabolic), 3) Assess compensation, 4) Determine if compensation is acute or chronic, 5) Calculate the anion gap, 6) If elevated, calculate the delta gap, 7) Consider differentials based on clinical context and lab results. Examples are provided to demonstrate applying the steps to analyze specific acid-base disorders like metabolic acidosis or alkalosis.




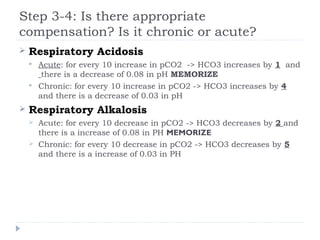
![Step 3-4: Is there appropriate
compensation? Is it acute or chronic ?
Metabolic Acidosis
Winter’s formula: pCO2 = 1.5[HCO3] + 8 ± 2 MEMORIZE
If serum pCO2 > expected pCO2 -> additional respiratory
acidosis
Metabolic Alkalosis
For every 10 increase in HCO3 -> pCO2 increases by 6](https://image.slidesharecdn.com/abgs-130804133323-phpapp01/85/Ab-gs-10-320.jpg)

![Step 5: Calculate the anion gap
AG = Na – Cl – HCO3 (normal 12 ± 2)
AG corrected = AG + 2.5[4 – albumin]
If there is an anion Gap then calculate the
Delta/delta gap (step 6). Only need to calculate
delta gap (excess anion gap) when there is an anion
gap to determine additional hidden metabolic
disorders (nongap metabolic acidosis or metabolic
alkalosis)
If there is no anion gap then start analyzing for
non-anion acidosis](https://image.slidesharecdn.com/abgs-130804133323-phpapp01/85/Ab-gs-12-320.jpg)