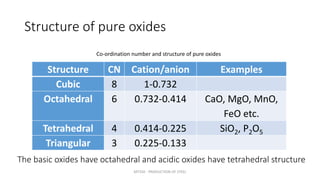
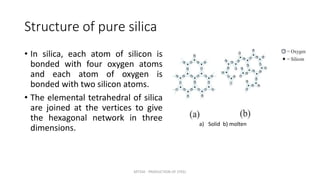
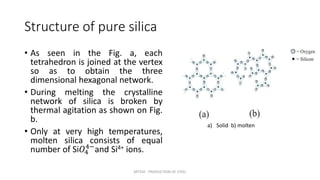
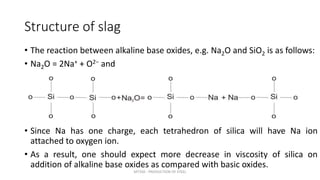
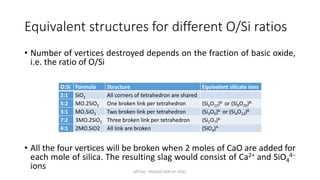
Slag plays an important role in steelmaking by acting as a sink for impurities and controlling the oxidizing potential of the steel. The structure of slag depends on the network former oxides like silica and network breaker oxides like calcium oxide. Pure silica has a tetrahedral structure that forms a hexagonal network, while basic oxides break this network by donating oxygen ions. The ratio of network former to breaker oxides determines how much of the silica network is broken, affecting the viscosity and properties of the slag. Slag is crucial for producing clean steel.