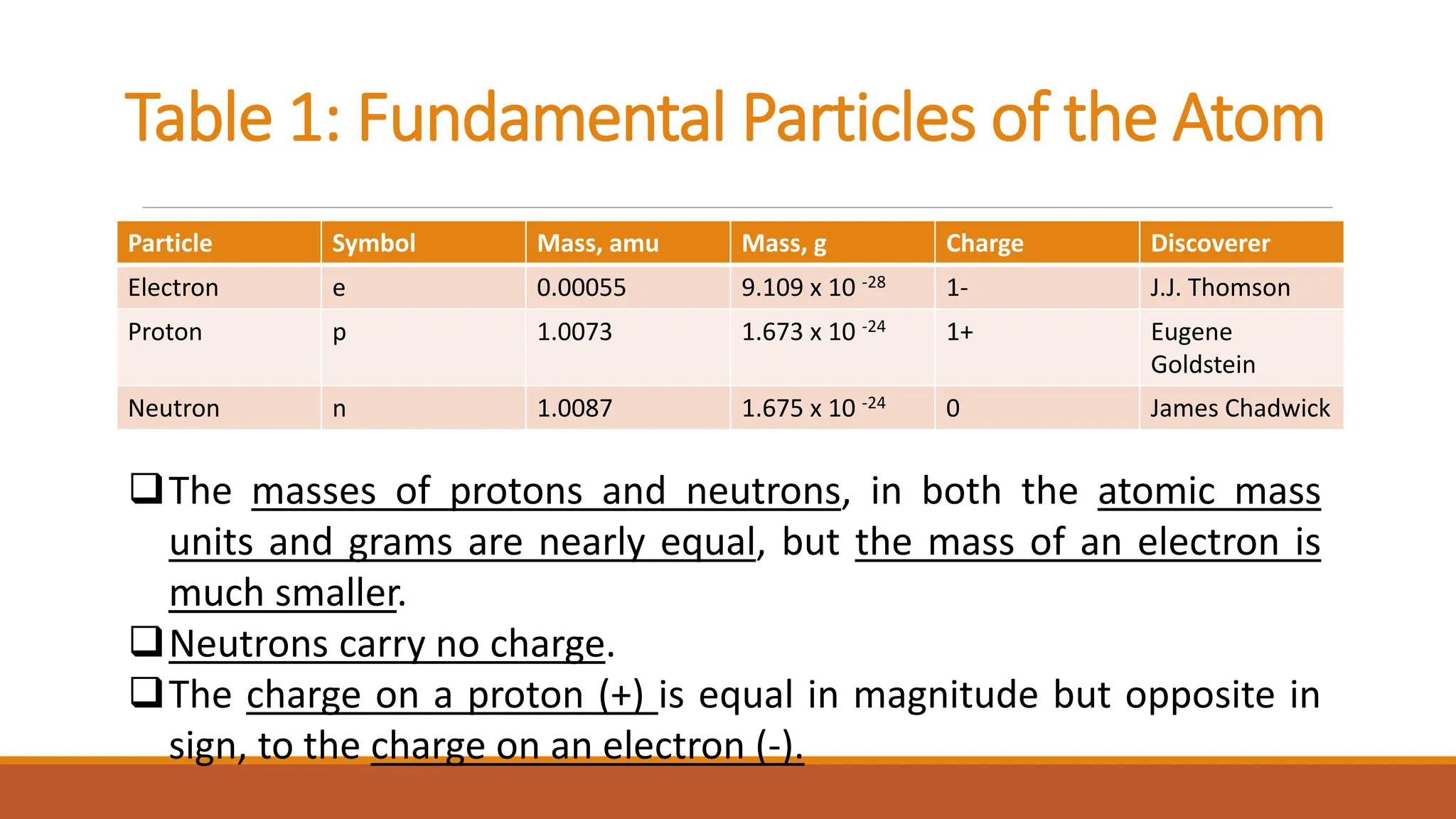
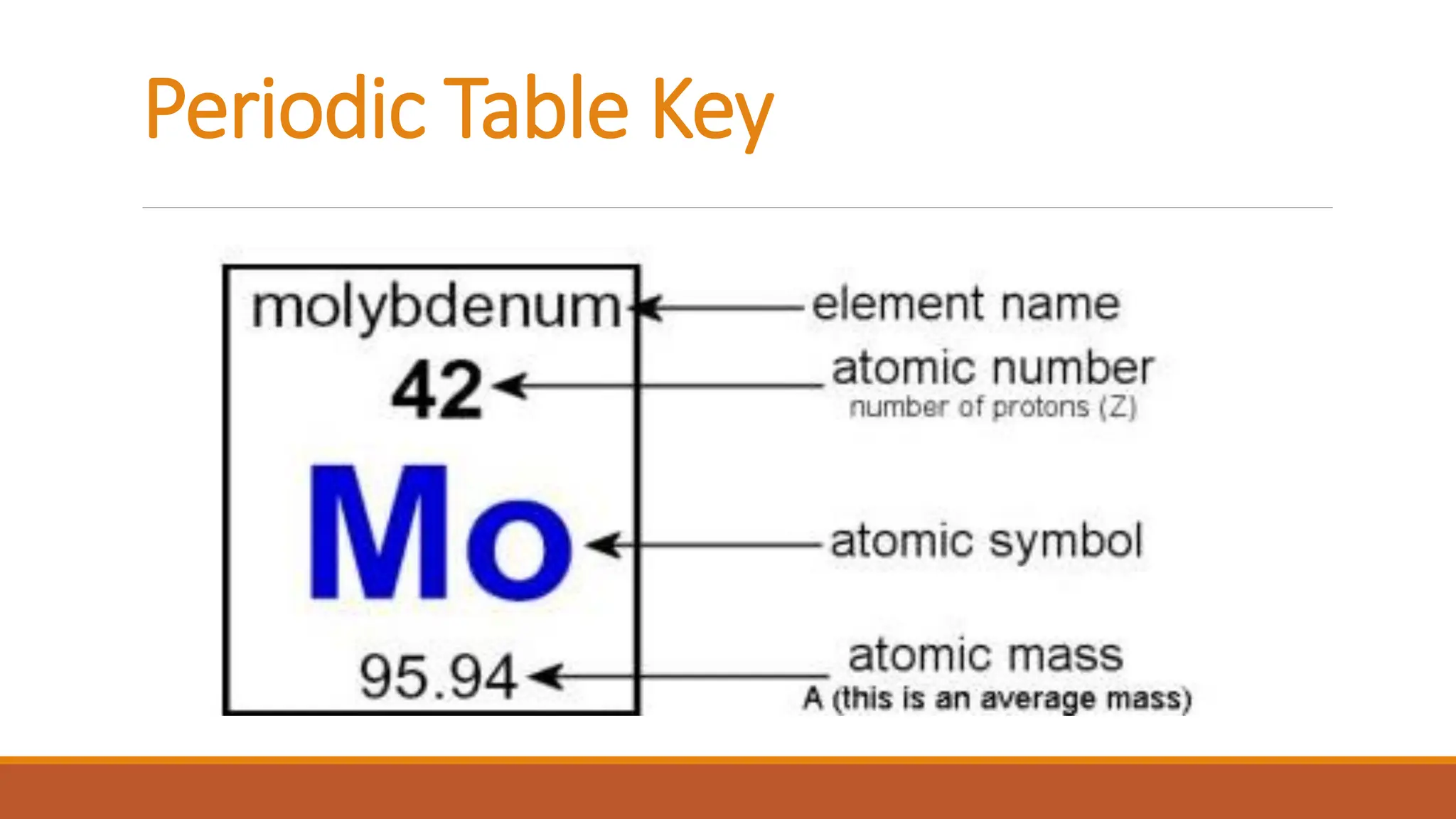
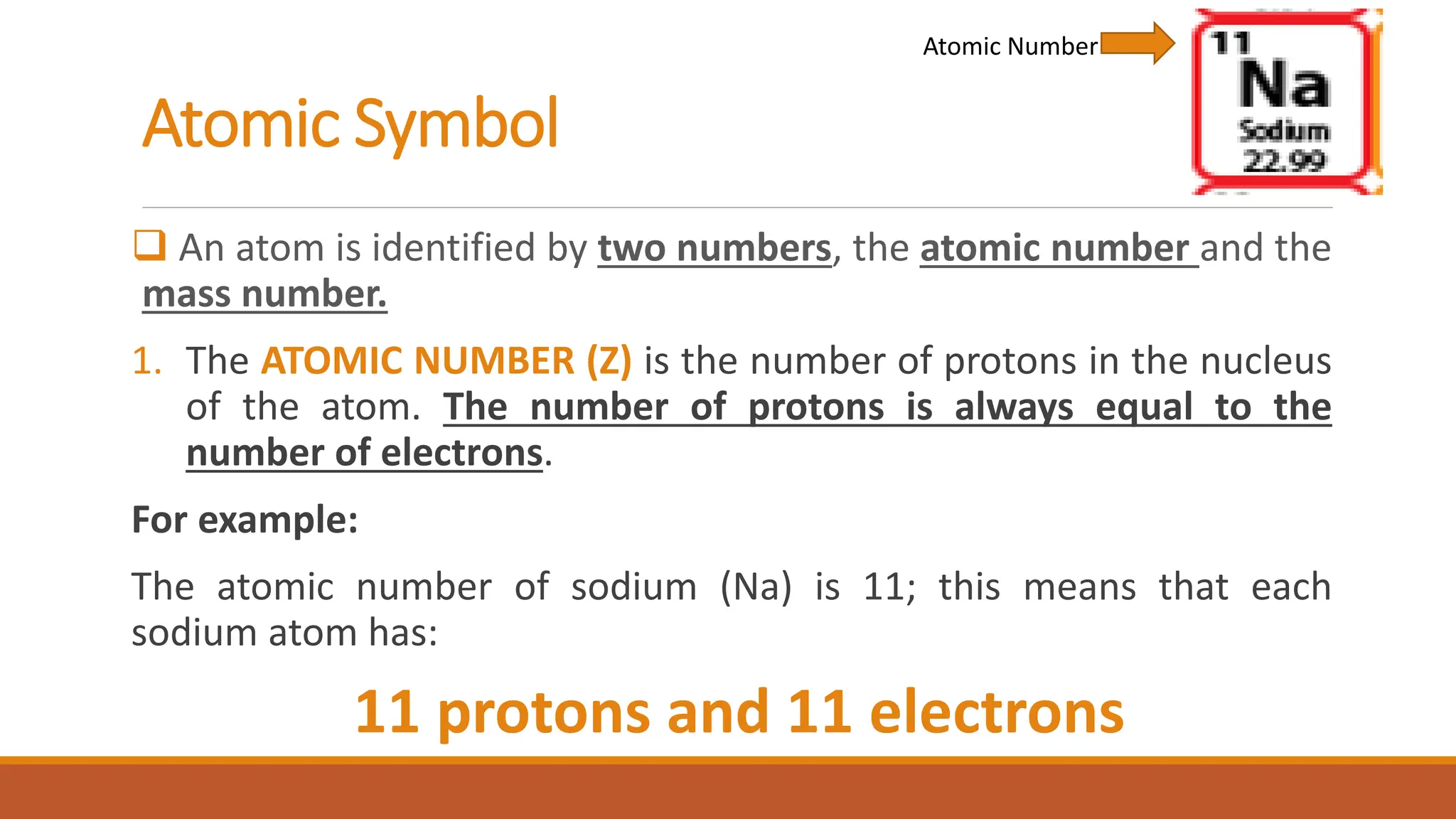
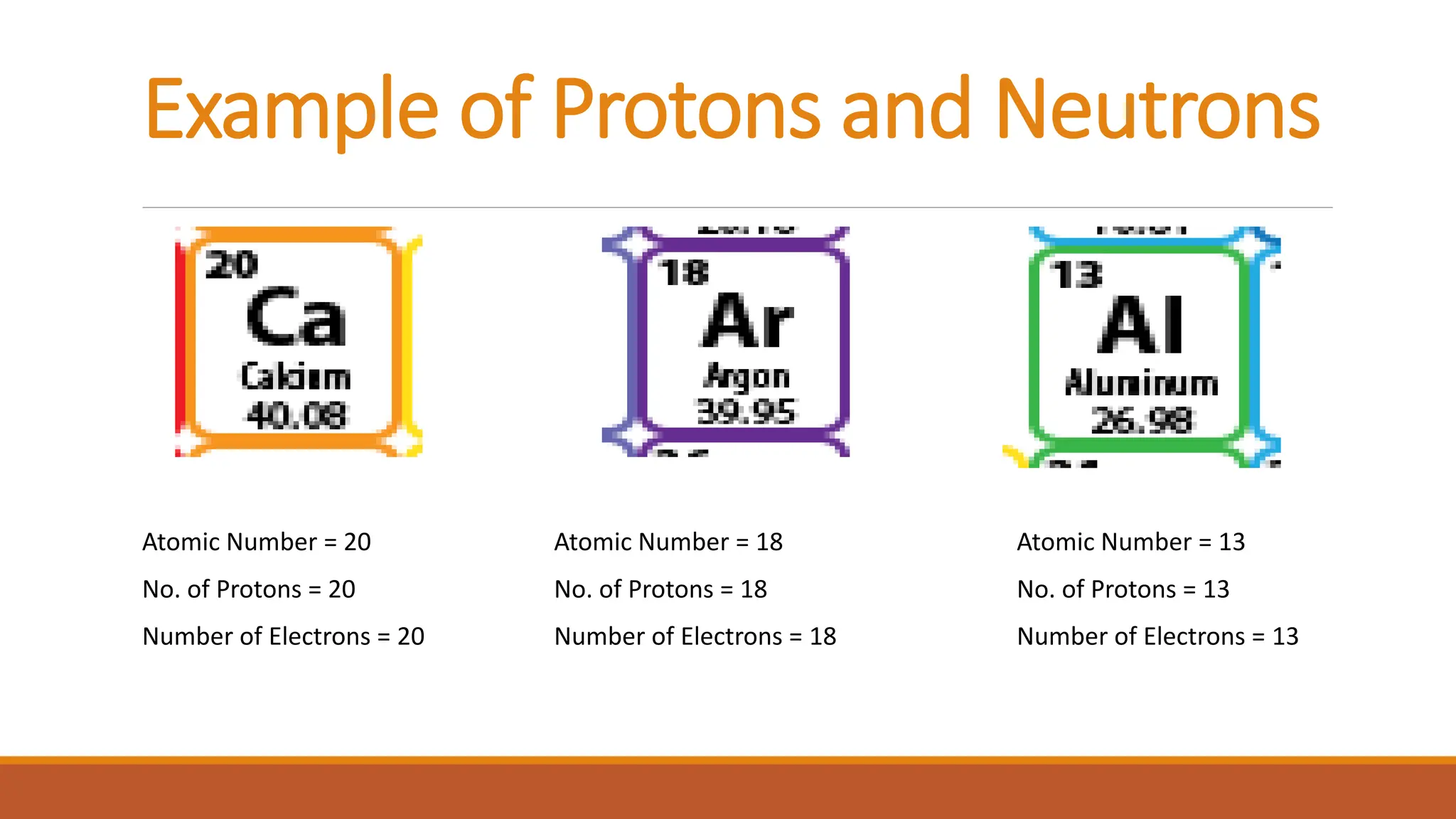
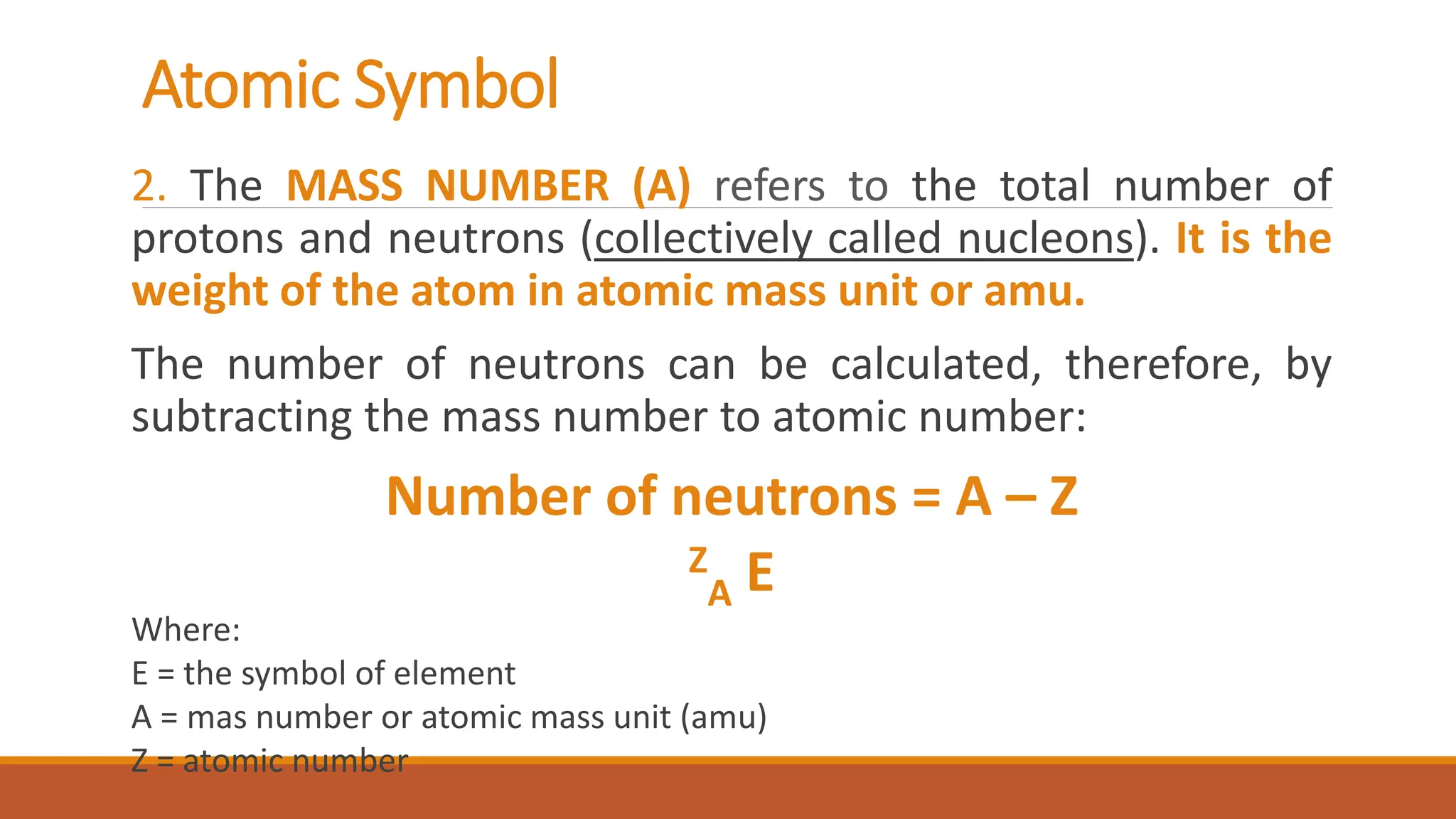
John Dalton was an English chemist known for developing modern atomic theory in 1808, proposing that matter consists of indivisible atoms that cannot be created or destroyed. Atoms are made up of electrons, protons, and neutrons, with the atomic number representing the number of protons and electrons in an atom. Dalton's theory marked a significant advancement in the understanding of chemistry, supported by earlier philosophical ideas from Democritus and subsequent experimental evidence.