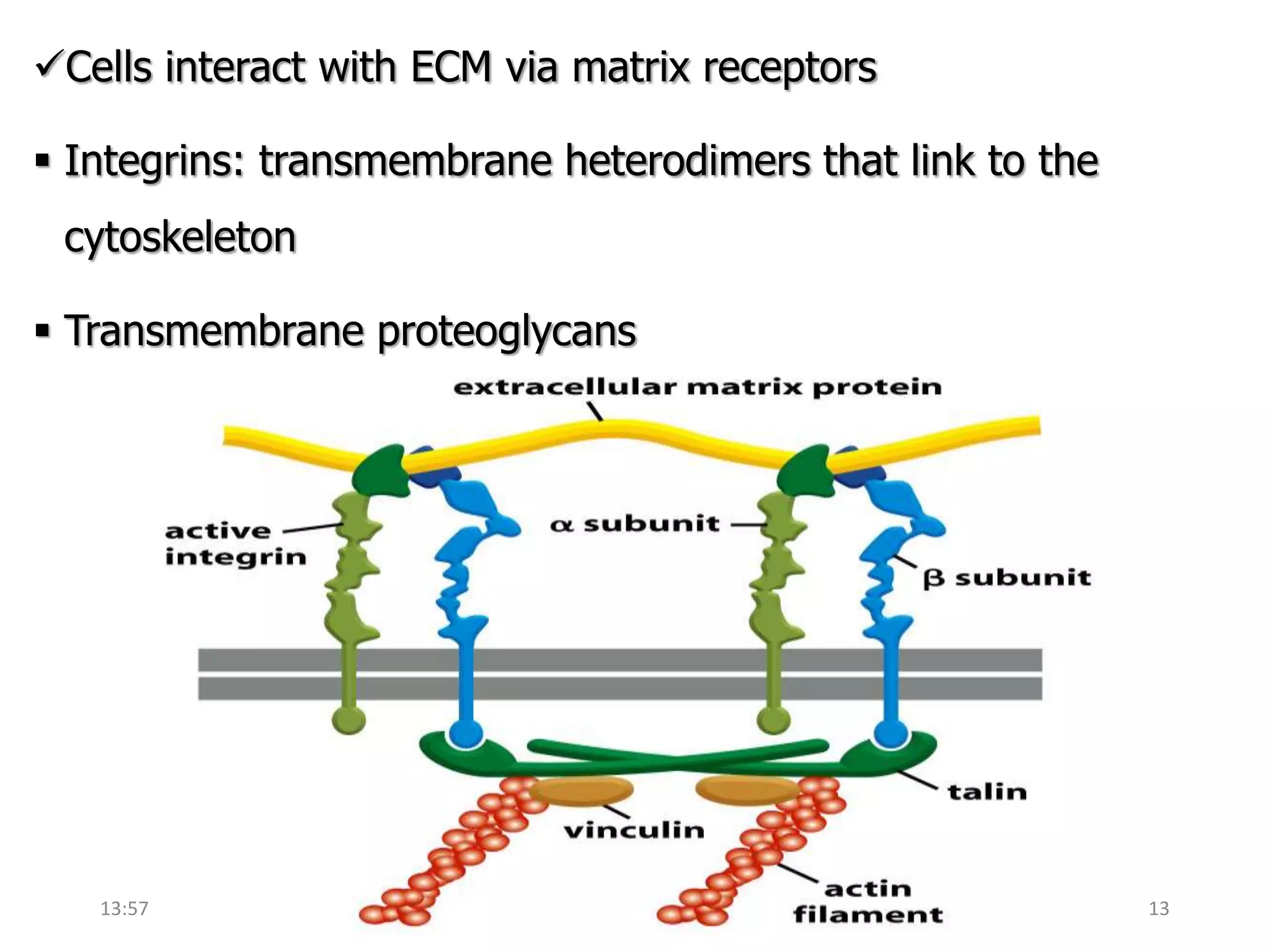
This document provides an overview of the extracellular matrix (ECM). It begins with an introduction defining the ECM and its main structural organizations of basal lamina and connective tissue. The main objectives and components of the ECM are then outlined, including glycosaminoglycans and fibrous proteins. Specific ECM components like hyaluronan, collagen, and elastin are described in more detail regarding their structure and function. Diseases associated with defects in ECM production and structure are also discussed.