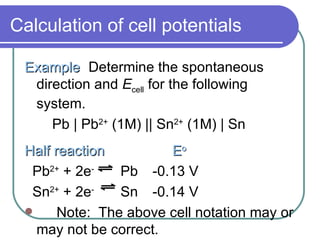
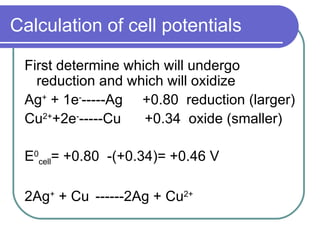
This document discusses half-cells, cell potentials, and how to calculate cell potentials using standard reduction potentials of half-reactions. The key points are:
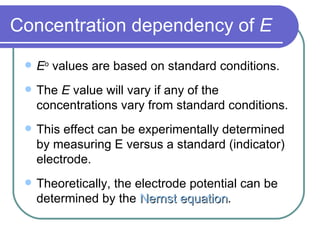
- Standard reduction potentials allow prediction of spontaneous reactions and equilibria.
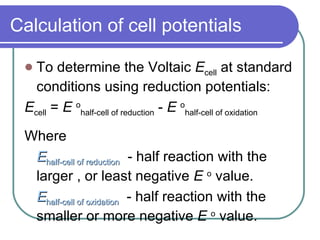
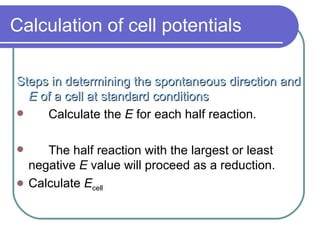
- Cell potentials (Ecell) are calculated as the potential of the reduction half-reaction minus the potential of the oxidation half-reaction.
- A positive Ecell indicates a spontaneous reaction with the half-reaction with the larger (least negative) potential proceeding as the reduction.