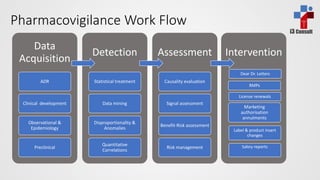
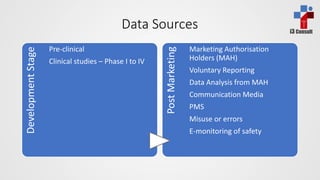
The document discusses pharmacovigilance, which involves monitoring the safety of medicinal products to detect and assess adverse effects. It explains the workflow for pharmacovigilance, including data acquisition, signal detection, and risk management, emphasizing the need for systematic processes and collaborative signal assessment. Additionally, it outlines the importance of risk management plans and strategies for risk reduction in specific sub-populations.


![i3 ConsultEverything in Pharma Has a System
According to the EMA:
A pharmacovigilance system is defined as a system used by an organisation to fulfil
its legal tasks and responsibilities in relation to pharmacovigilance and designed to
monitor the safety of authorized medicinal products and detect any change to their
risk-benefit balance [DIR Art 1(28d)].
A pharmacovigilance system, like any system, is characterised by its structures,
processes and outcomes. For each specific pharmacovigilance process, including its
necessary structures, a dedicated Module is included in good pharmacovigilance
practices (GVP).](https://image.slidesharecdn.com/pharmacovigilance-180807083217/85/Pharmacovigilance-3-320.jpg)