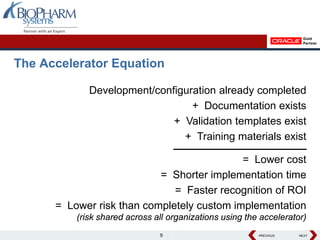
The document discusses the benefits of using a software accelerator for clinical trial management implementations. An accelerator bundles pre-built configurations, documentation, training materials and validation templates to streamline implementations. Case studies show accelerators can reduce implementation timelines from 1-2 years to 3 months and lower costs by avoiding customizations. Choosing an accelerator with proven features, flexibility and vendor support can help organizations implement clinical trial management systems faster and with lower risk.