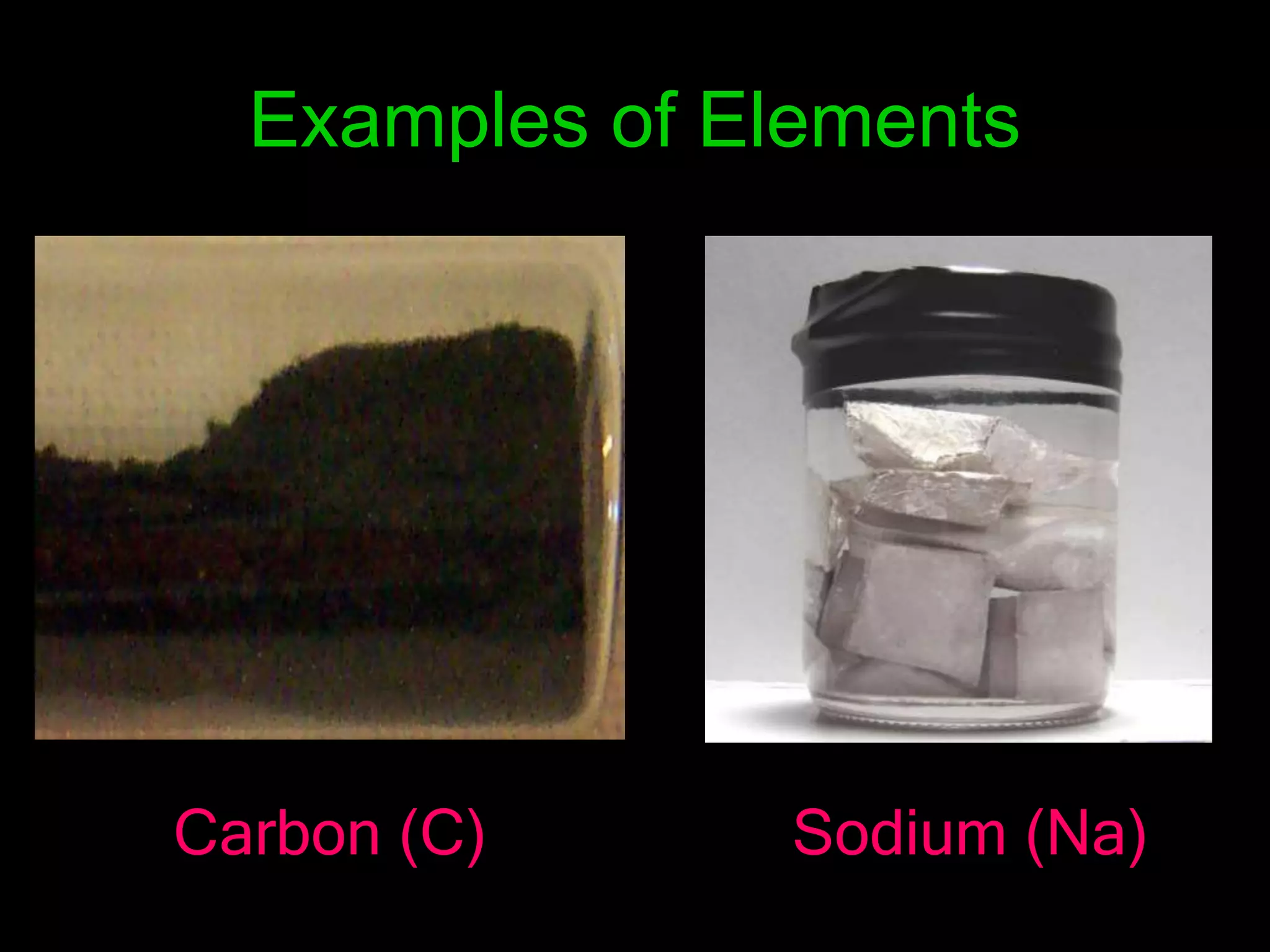
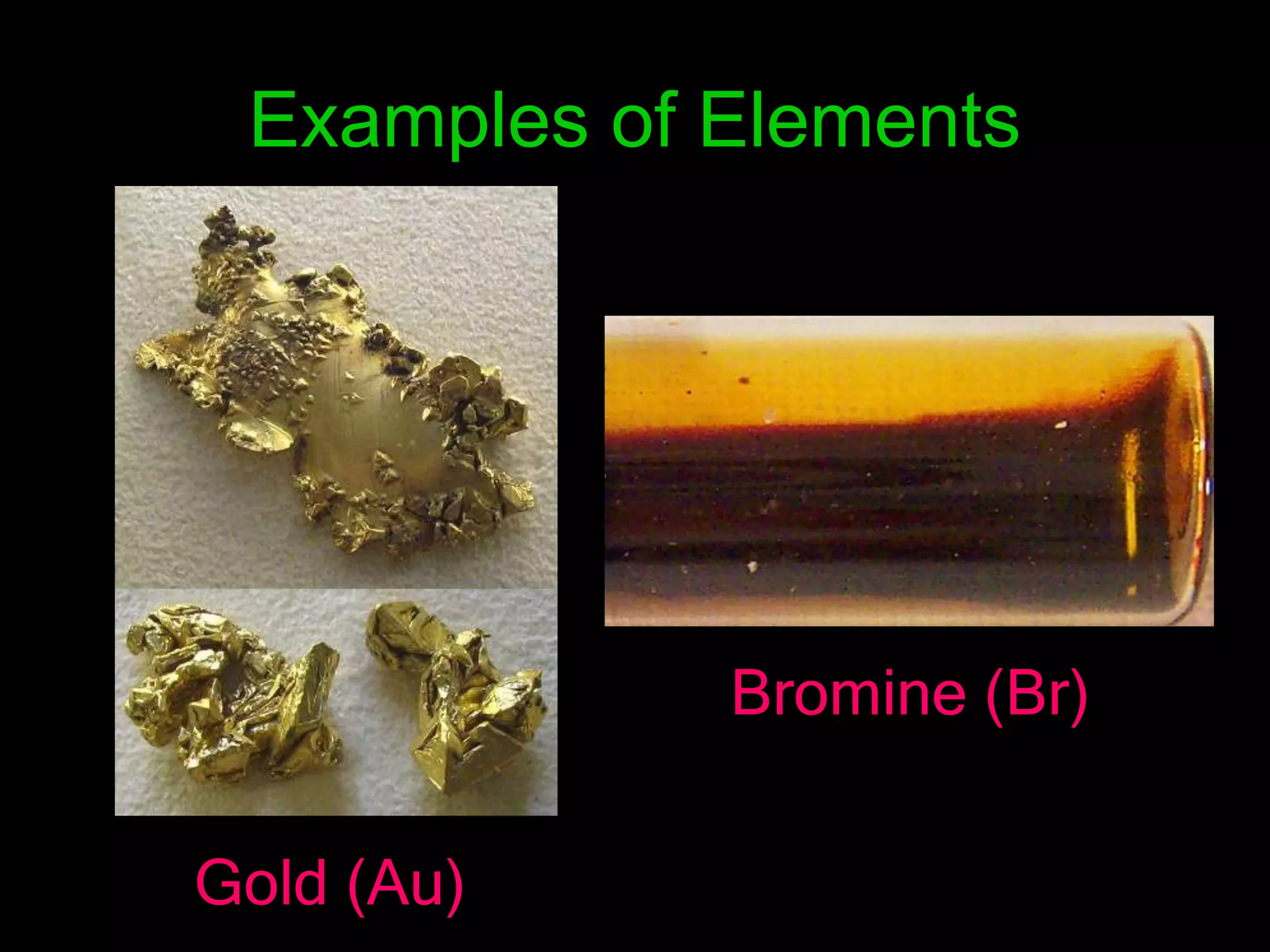
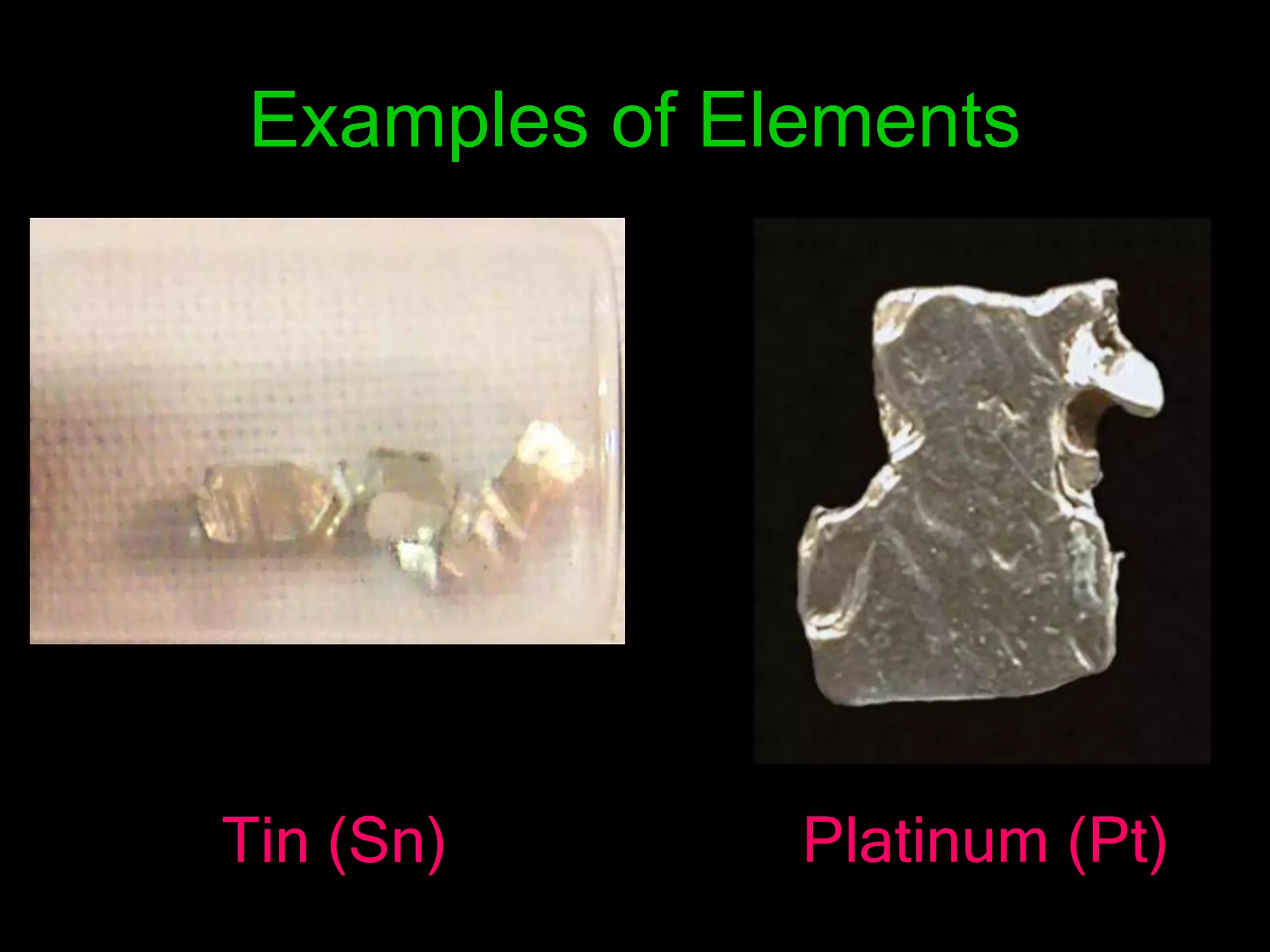
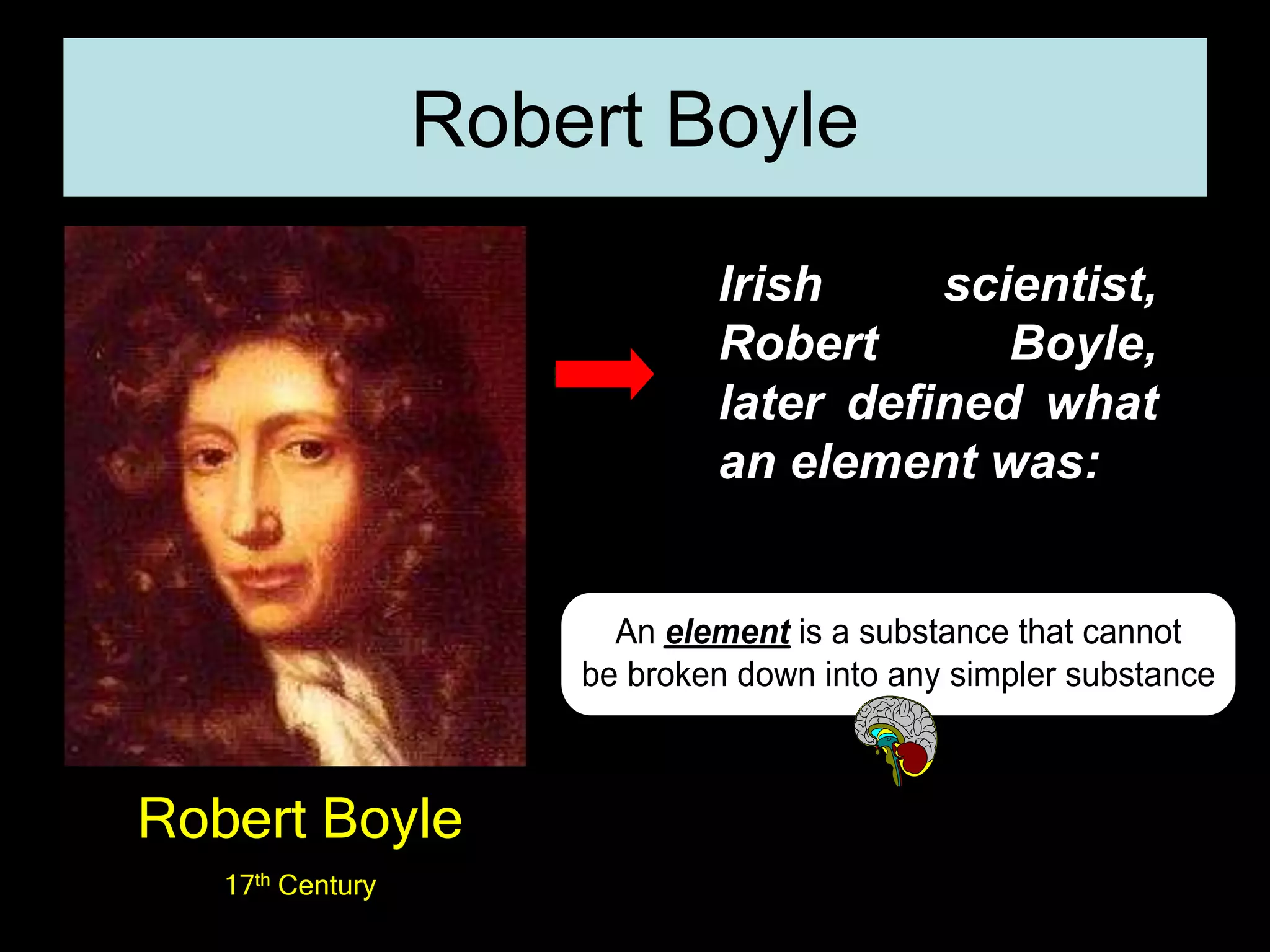
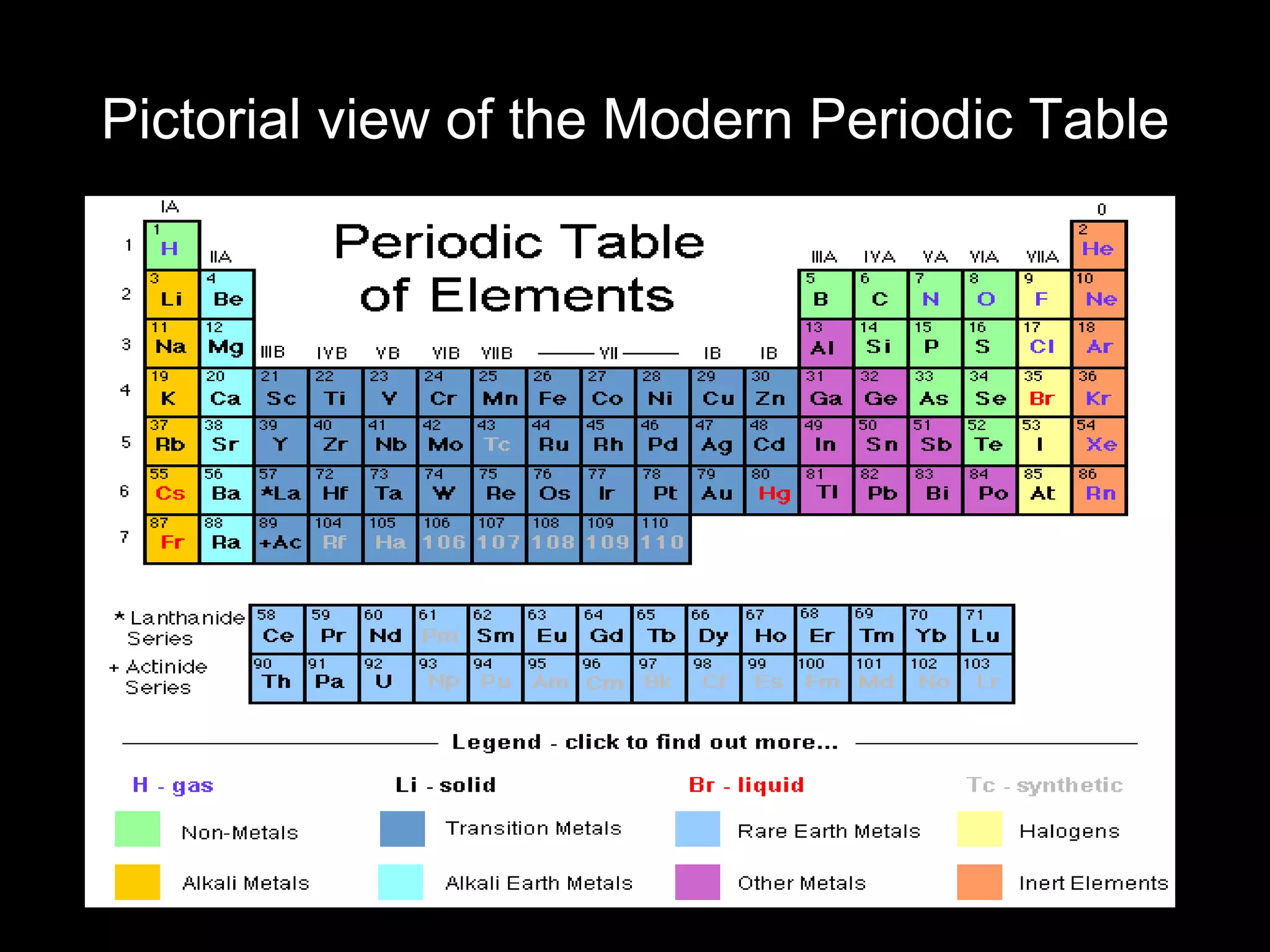
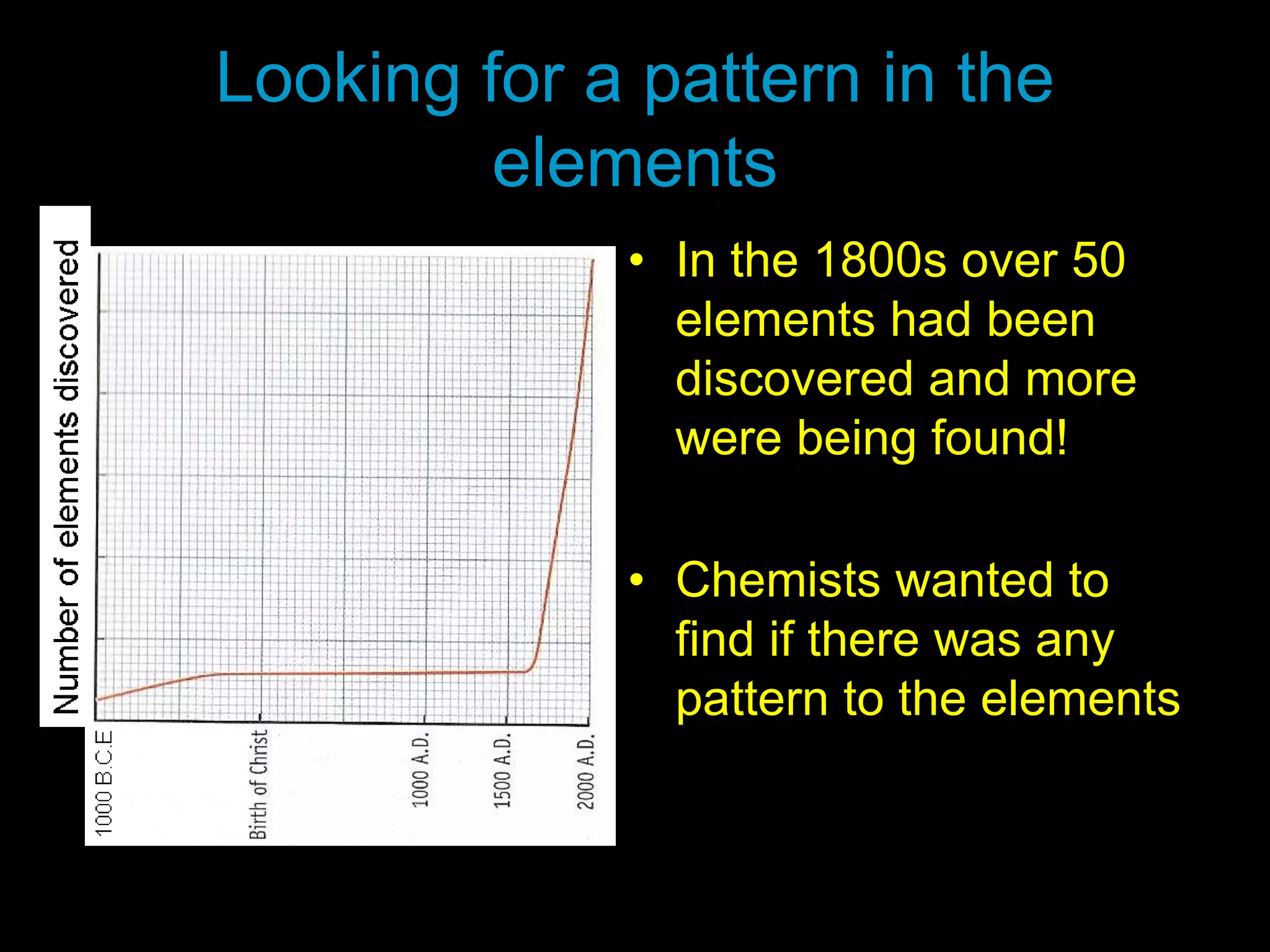
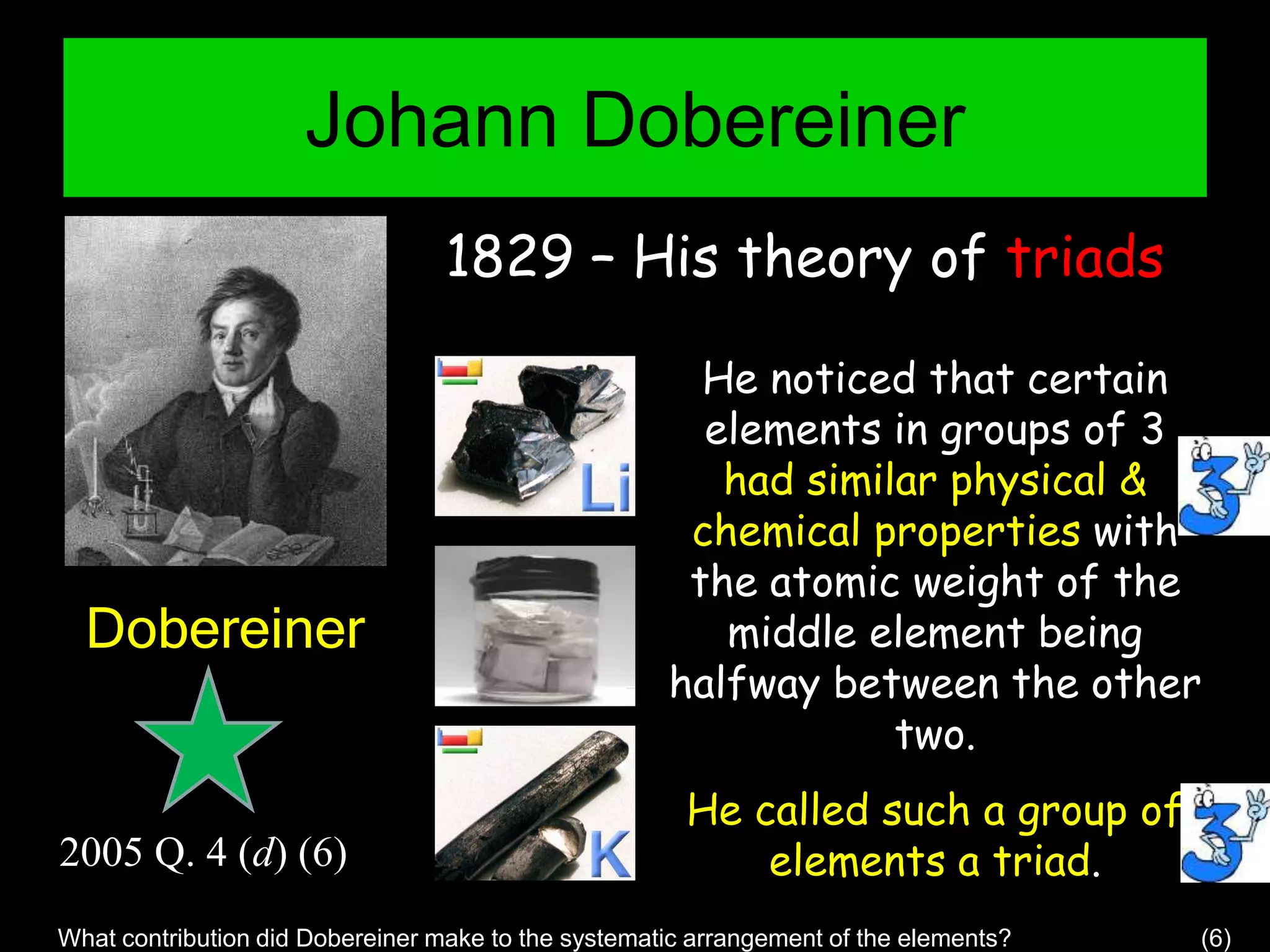
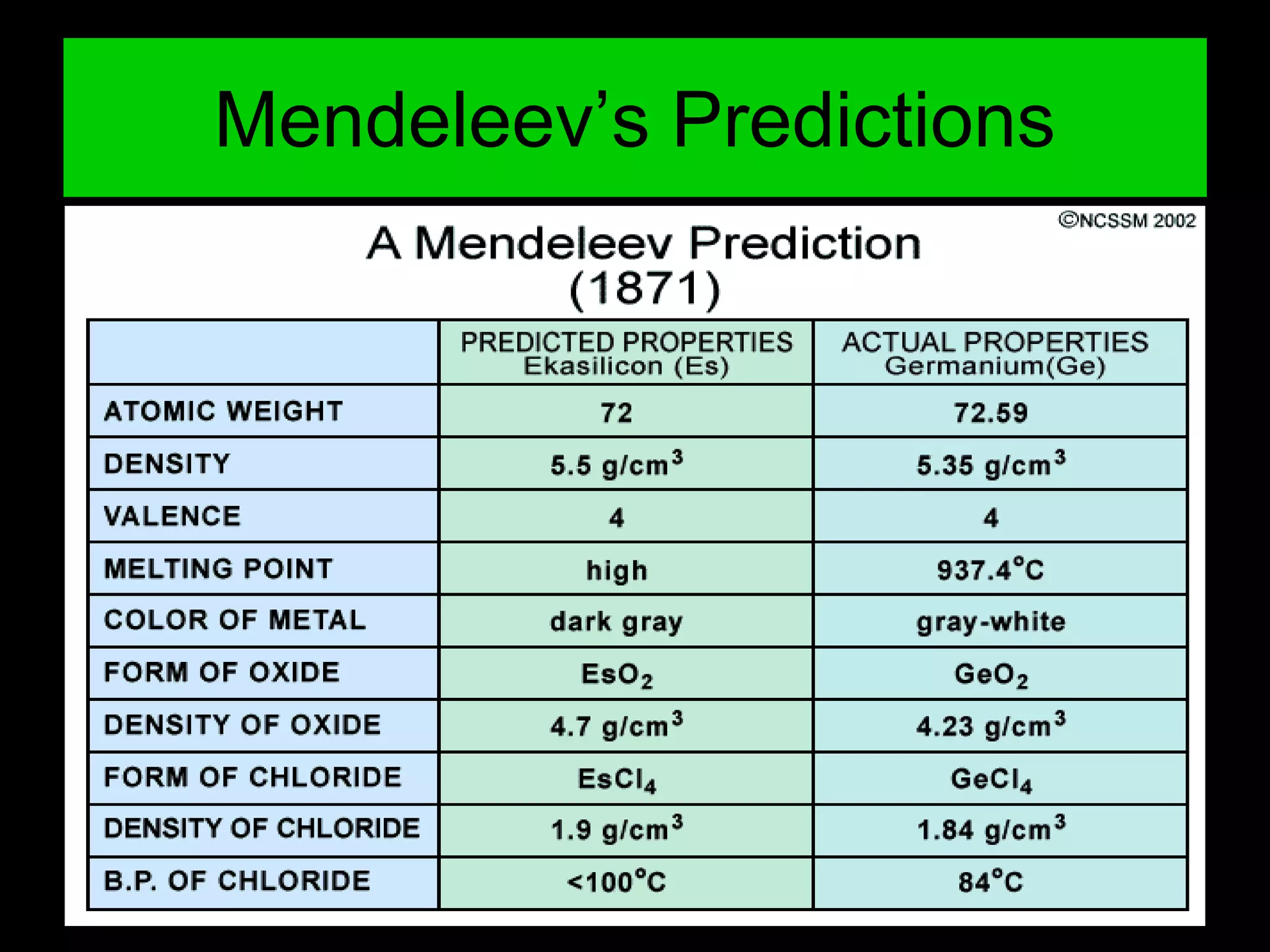
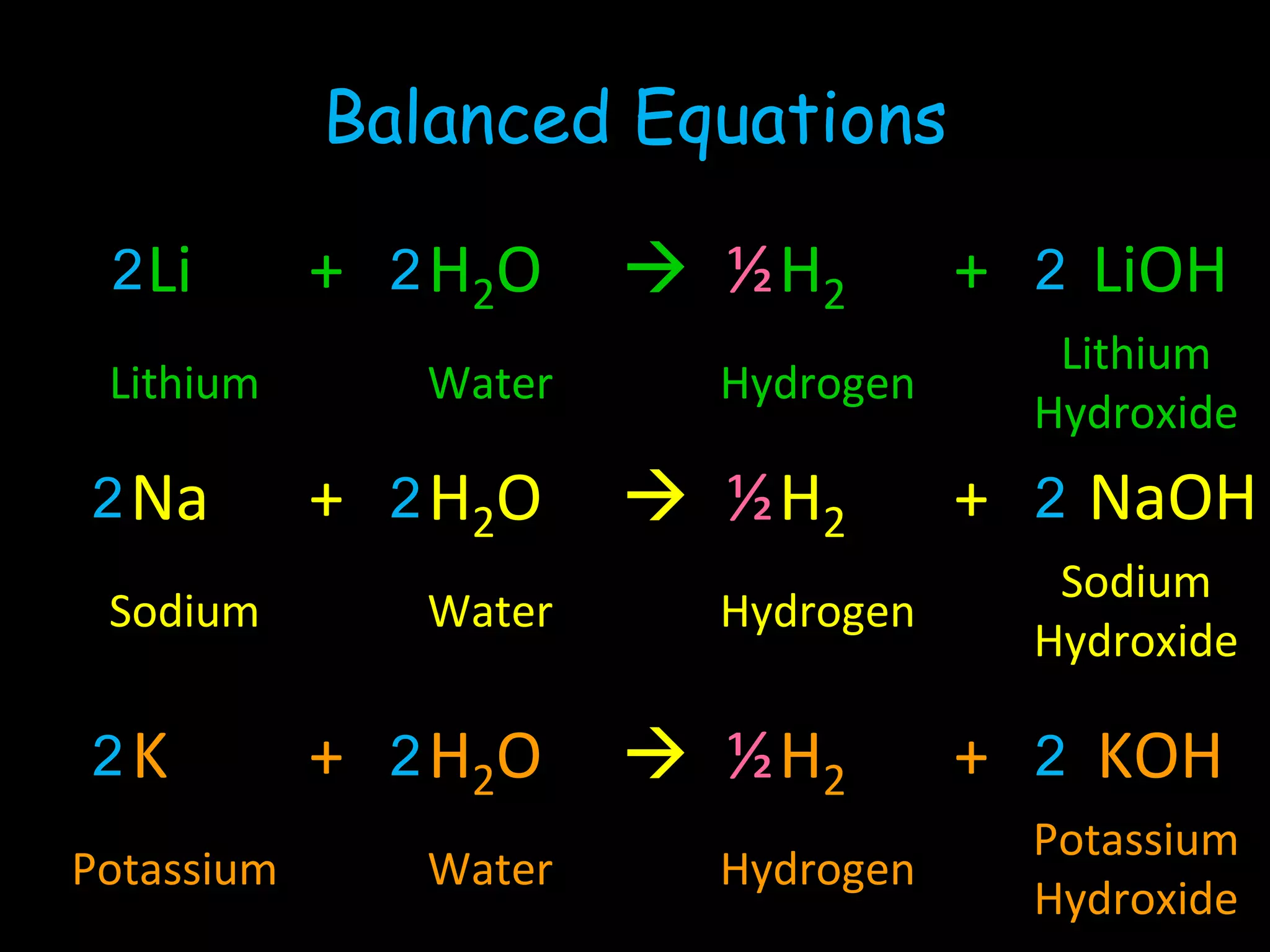
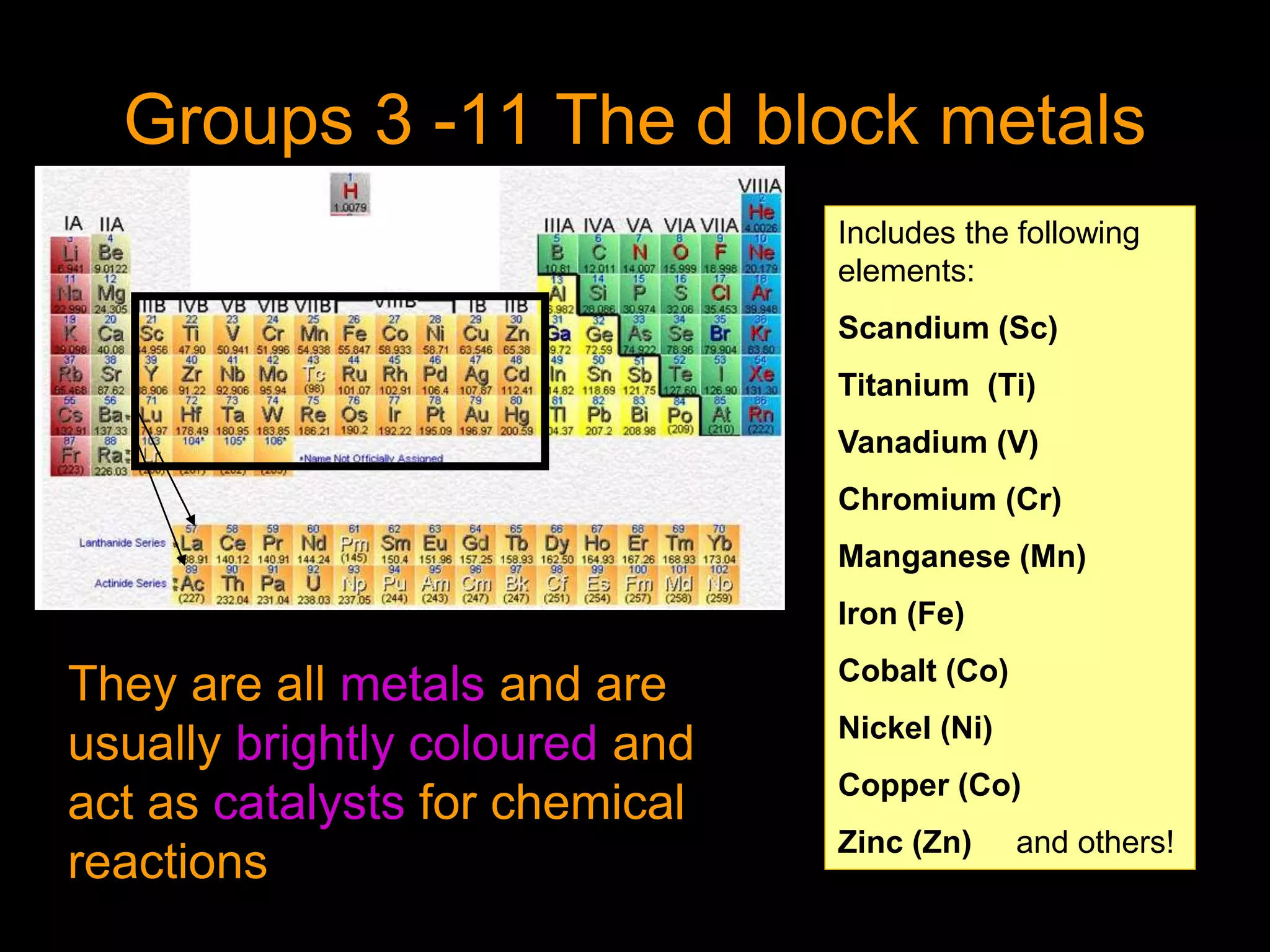
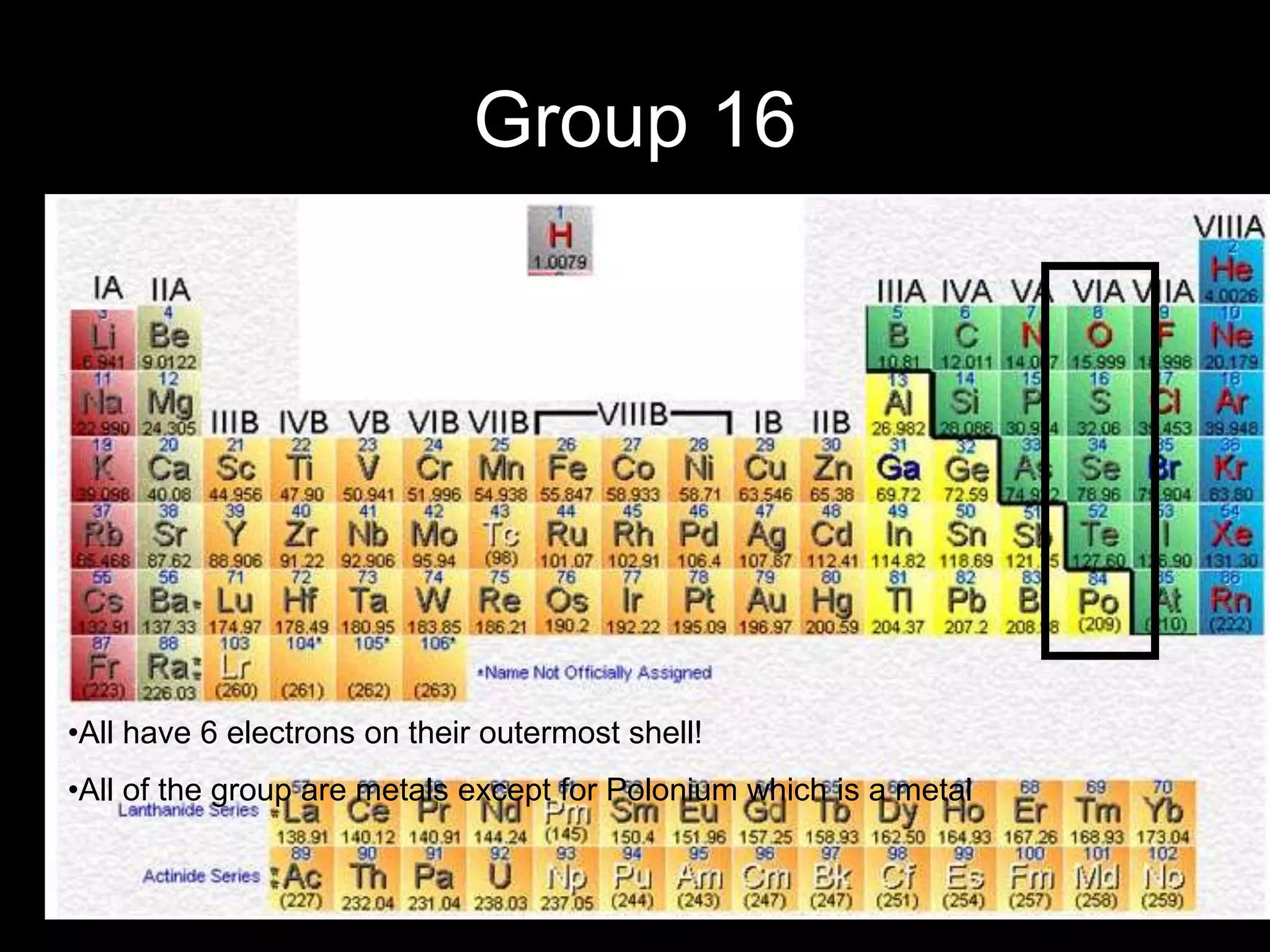
The document provides information about the history and development of the periodic table. It discusses how ancient Greeks like Empedocles believed that all matter was composed of four basic elements. It then outlines key contributions by Robert Boyle, Humphry Davy, Dobereiner, Newlands and Mendeleev to developing patterns in the properties of elements and early periodic table arrangements. Finally, it mentions Henry Moseley's discovery that atomic number, not mass, is the fundamental property determining an element's position in the periodic table.