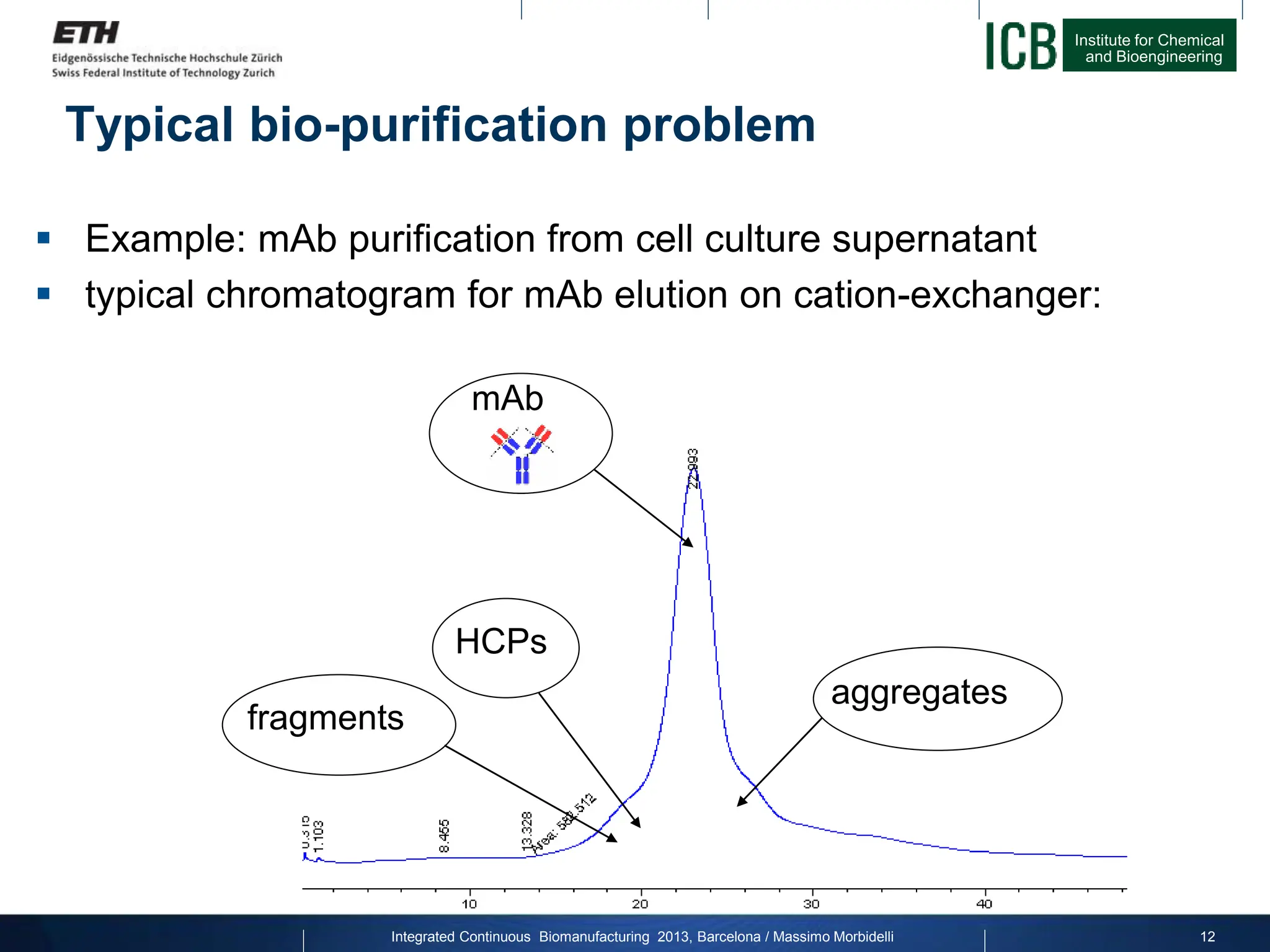
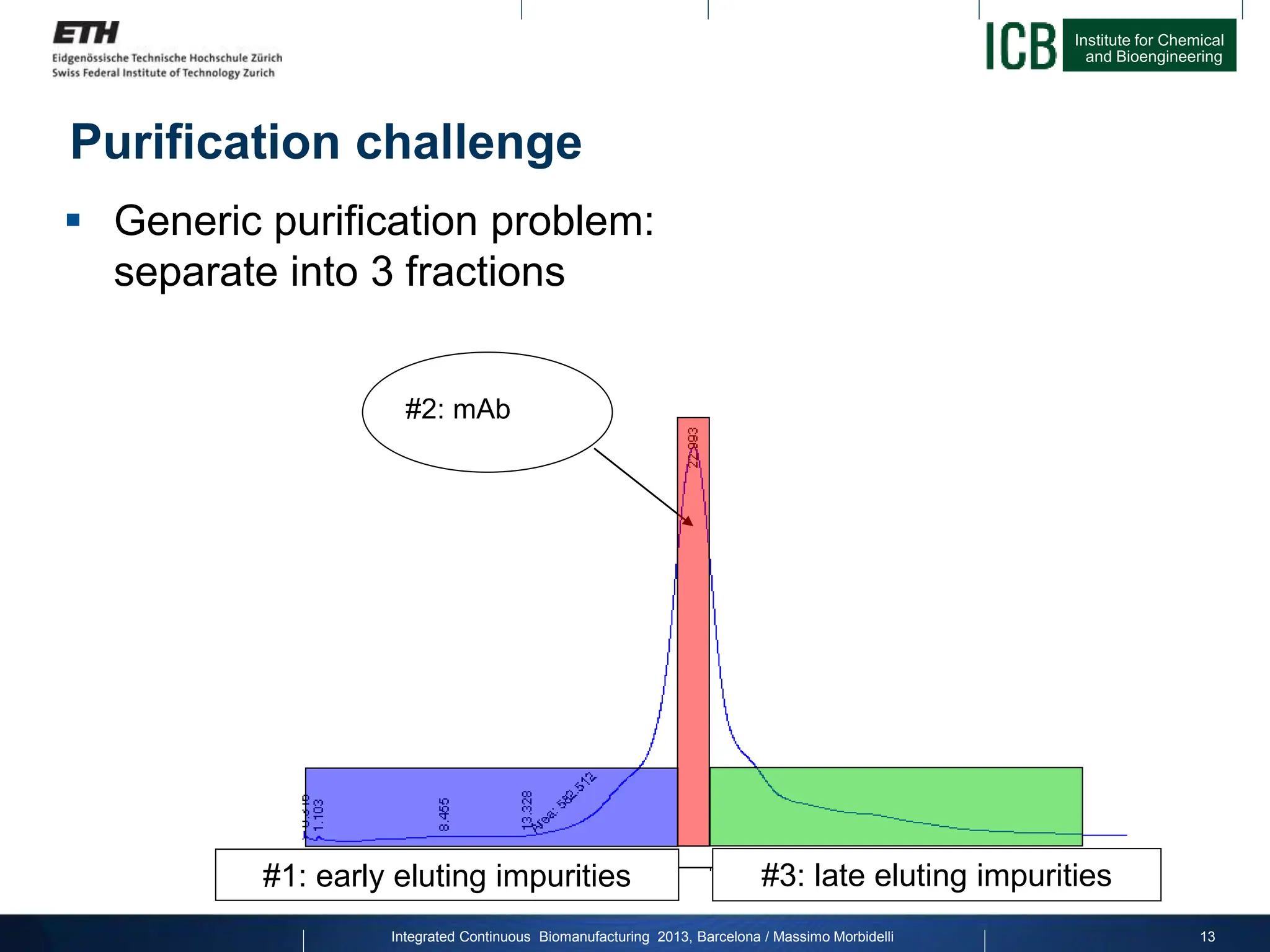
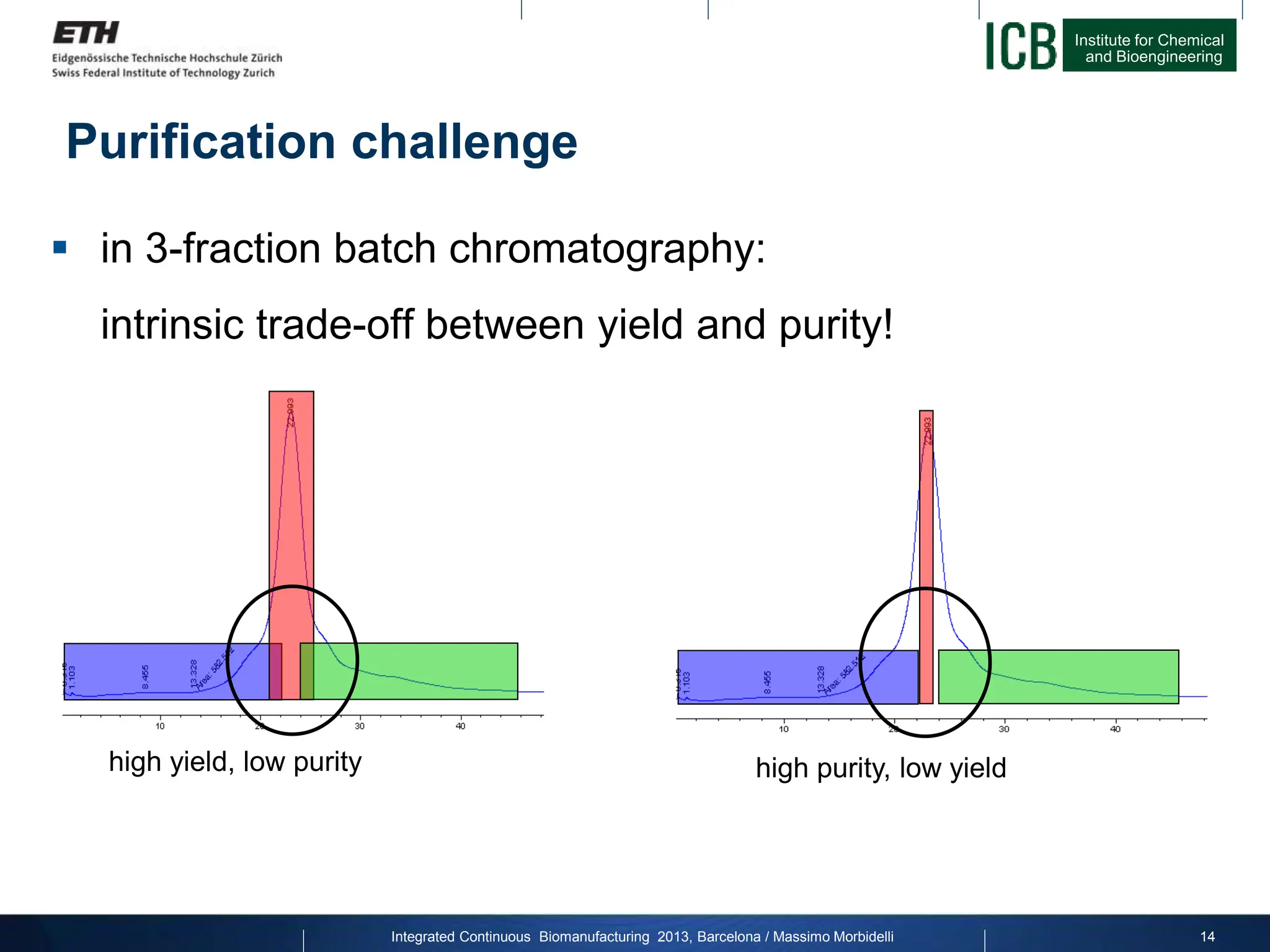
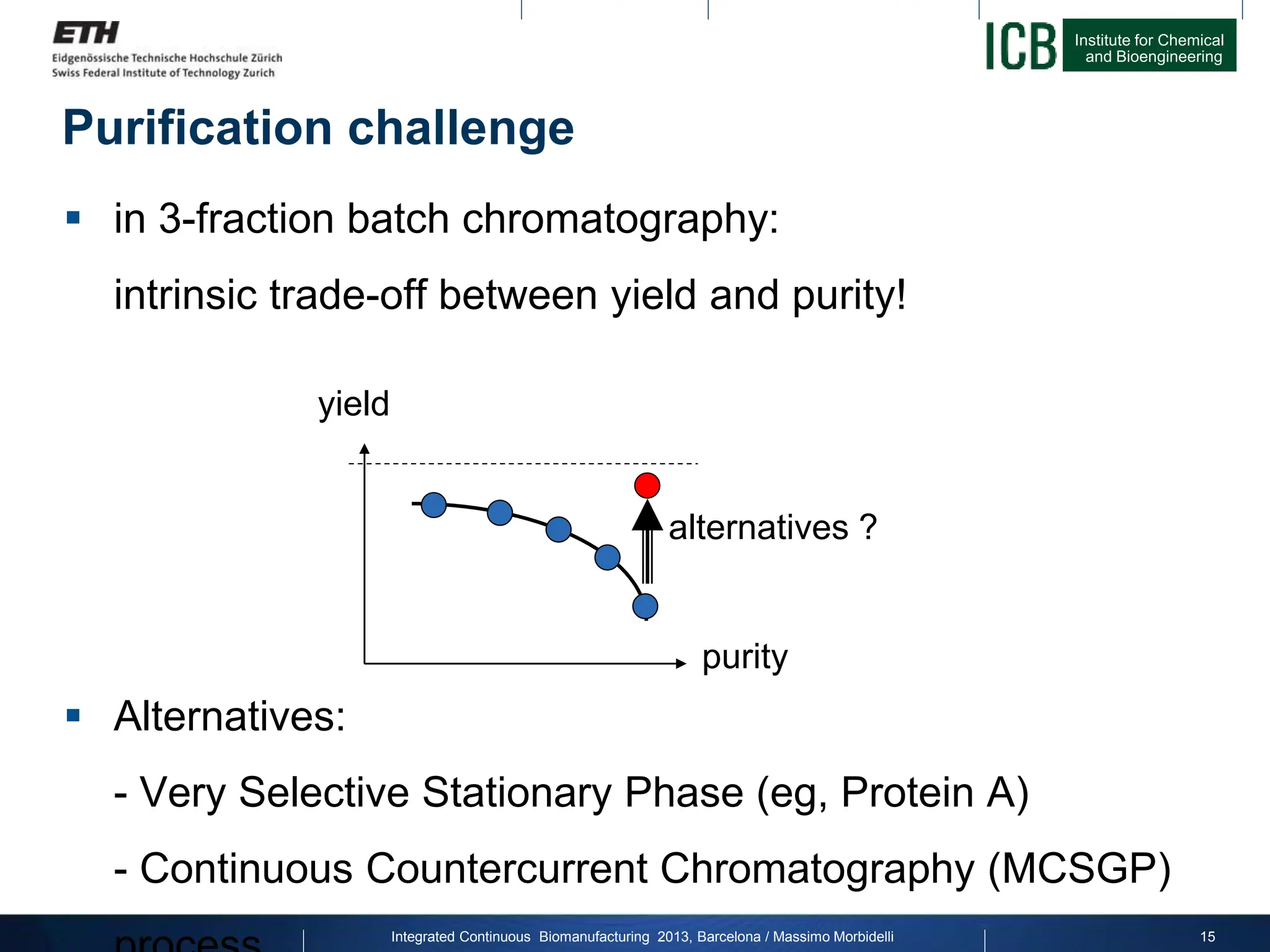
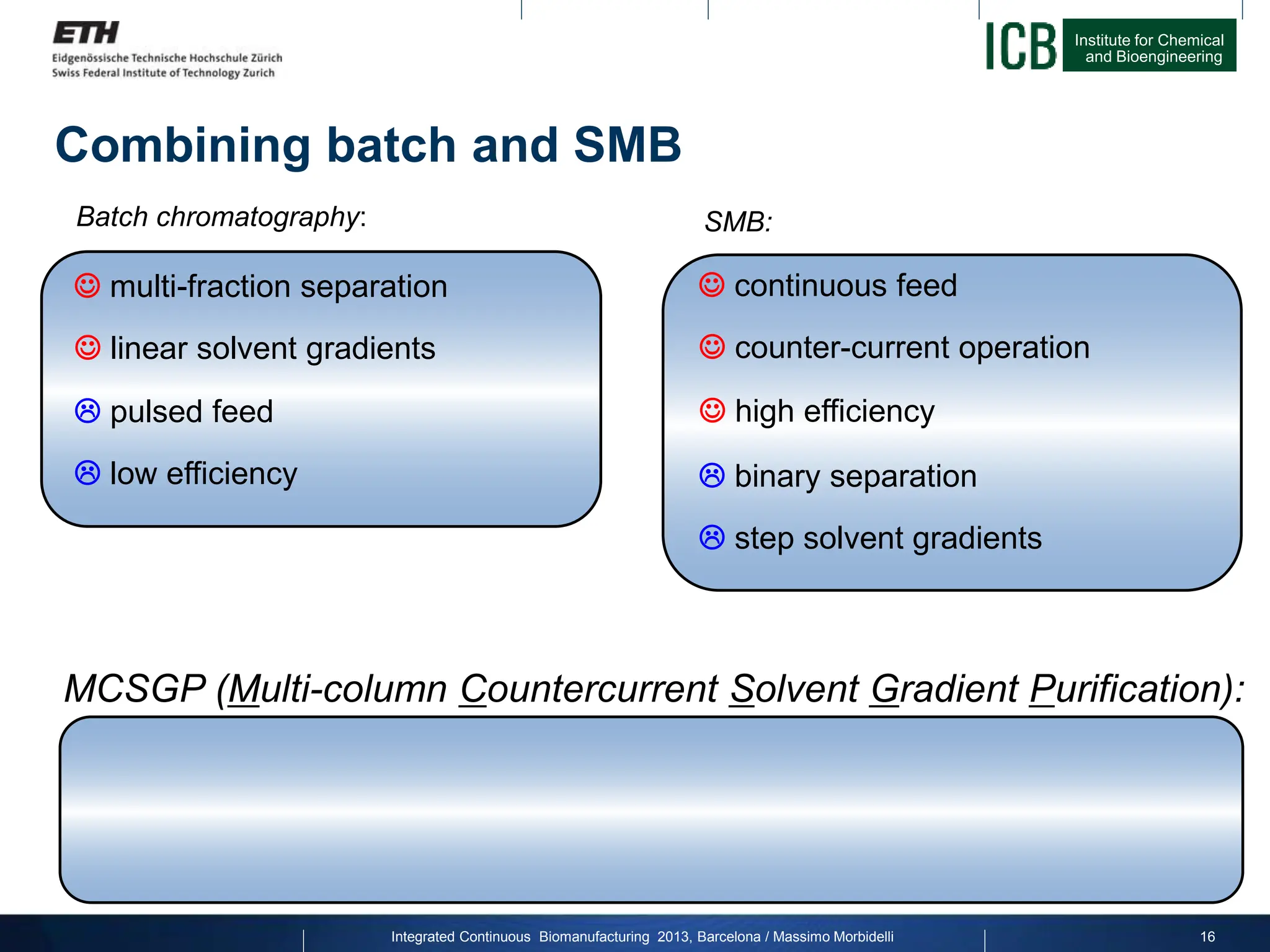
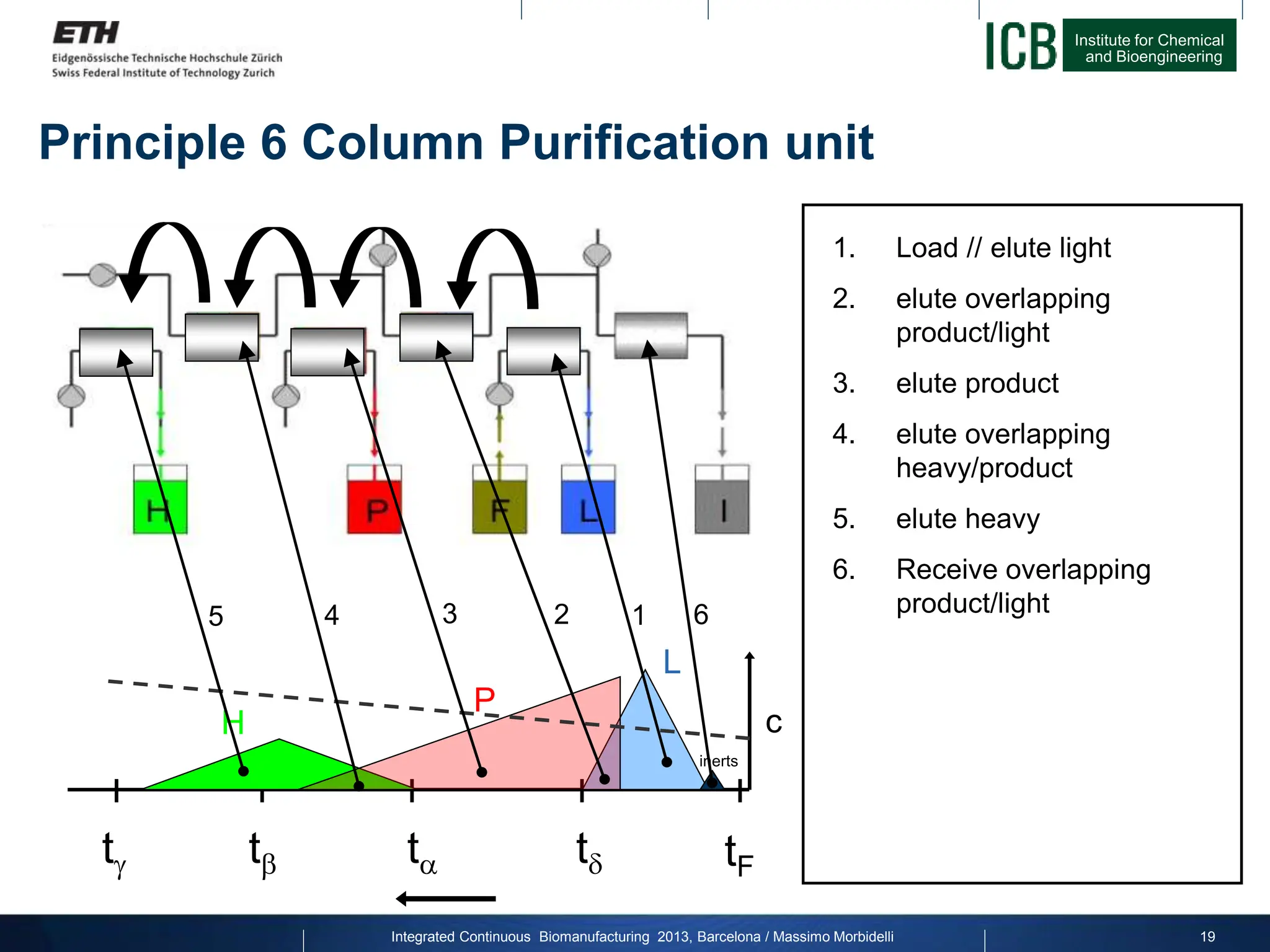
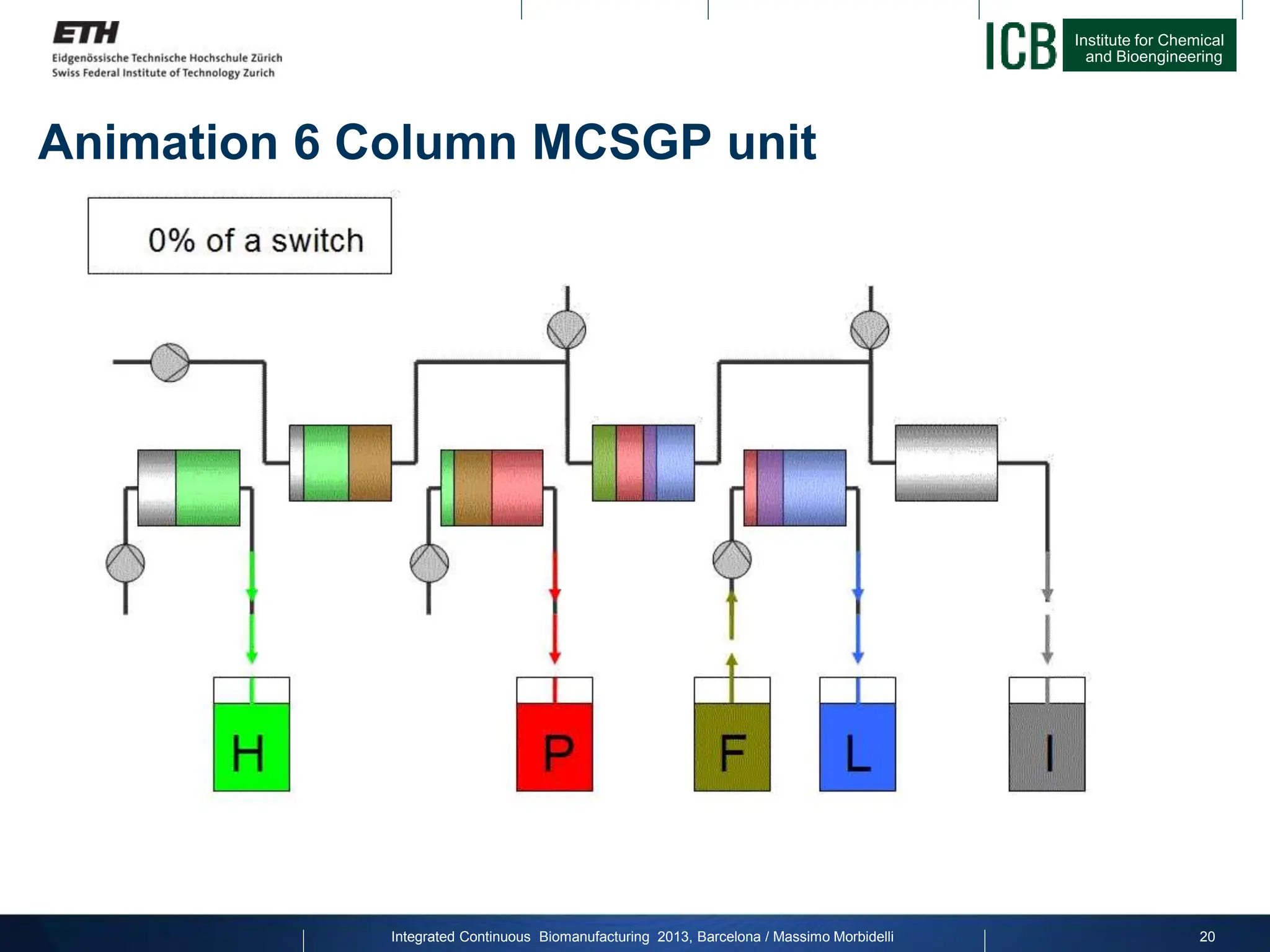
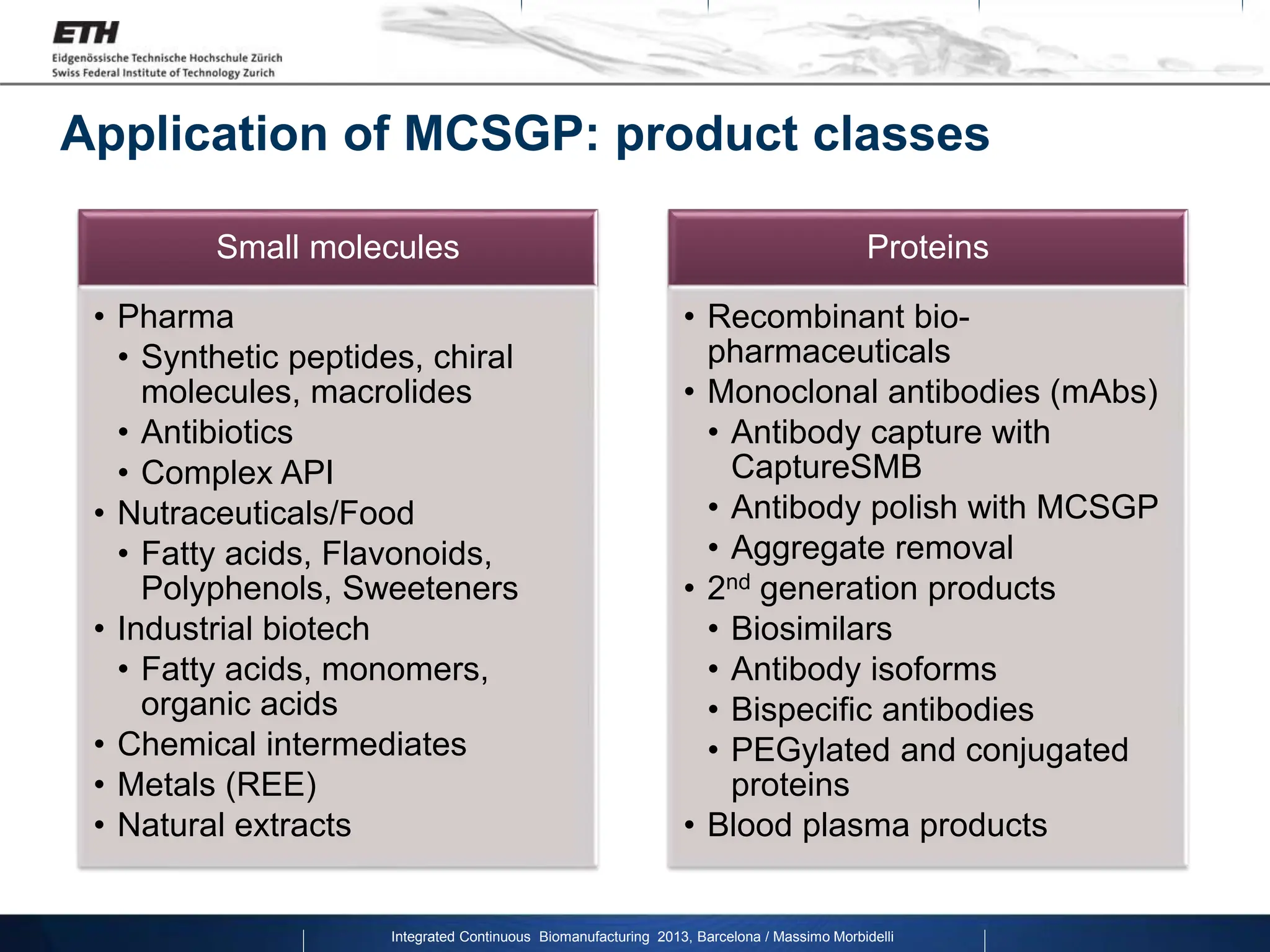
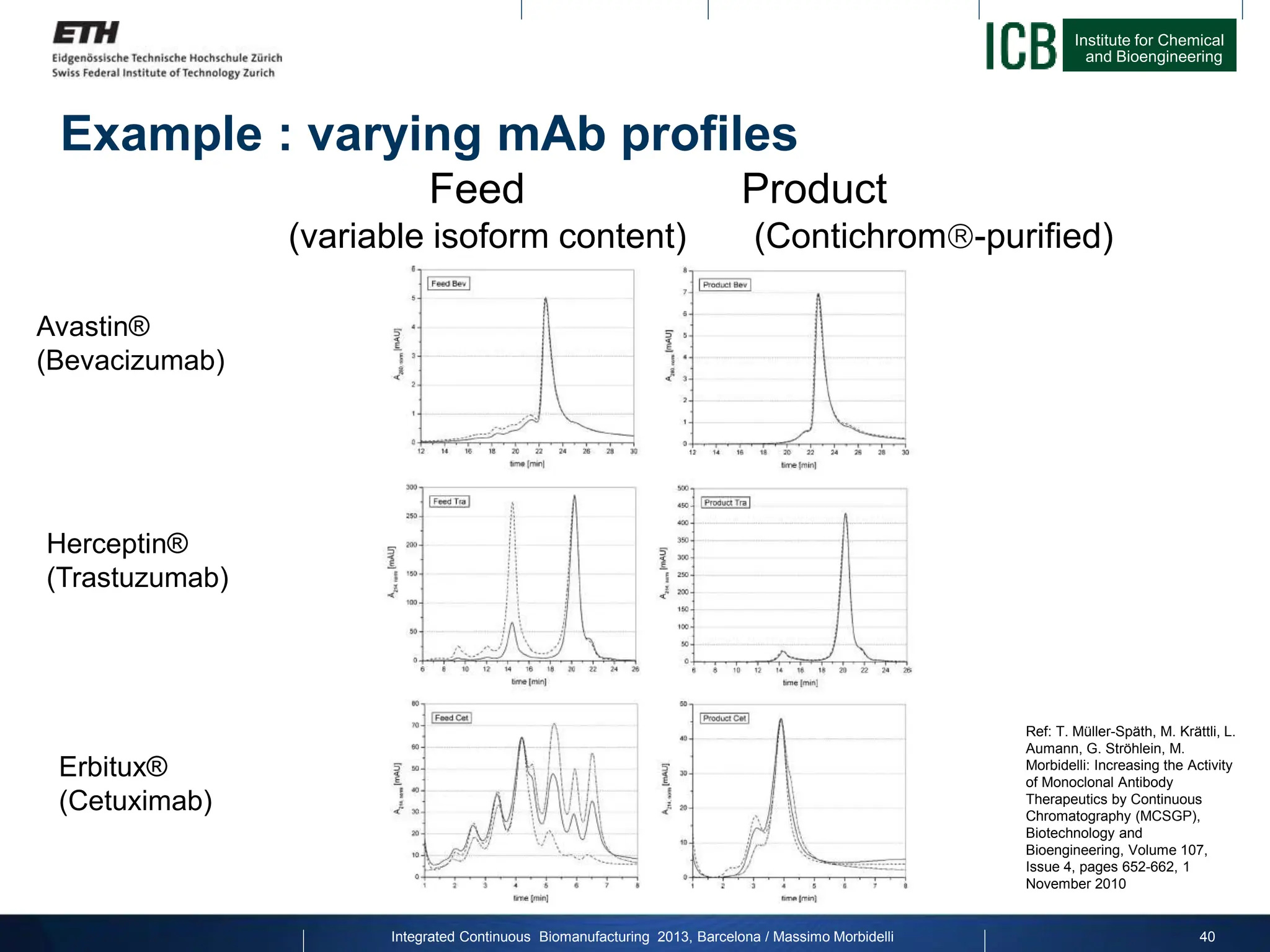
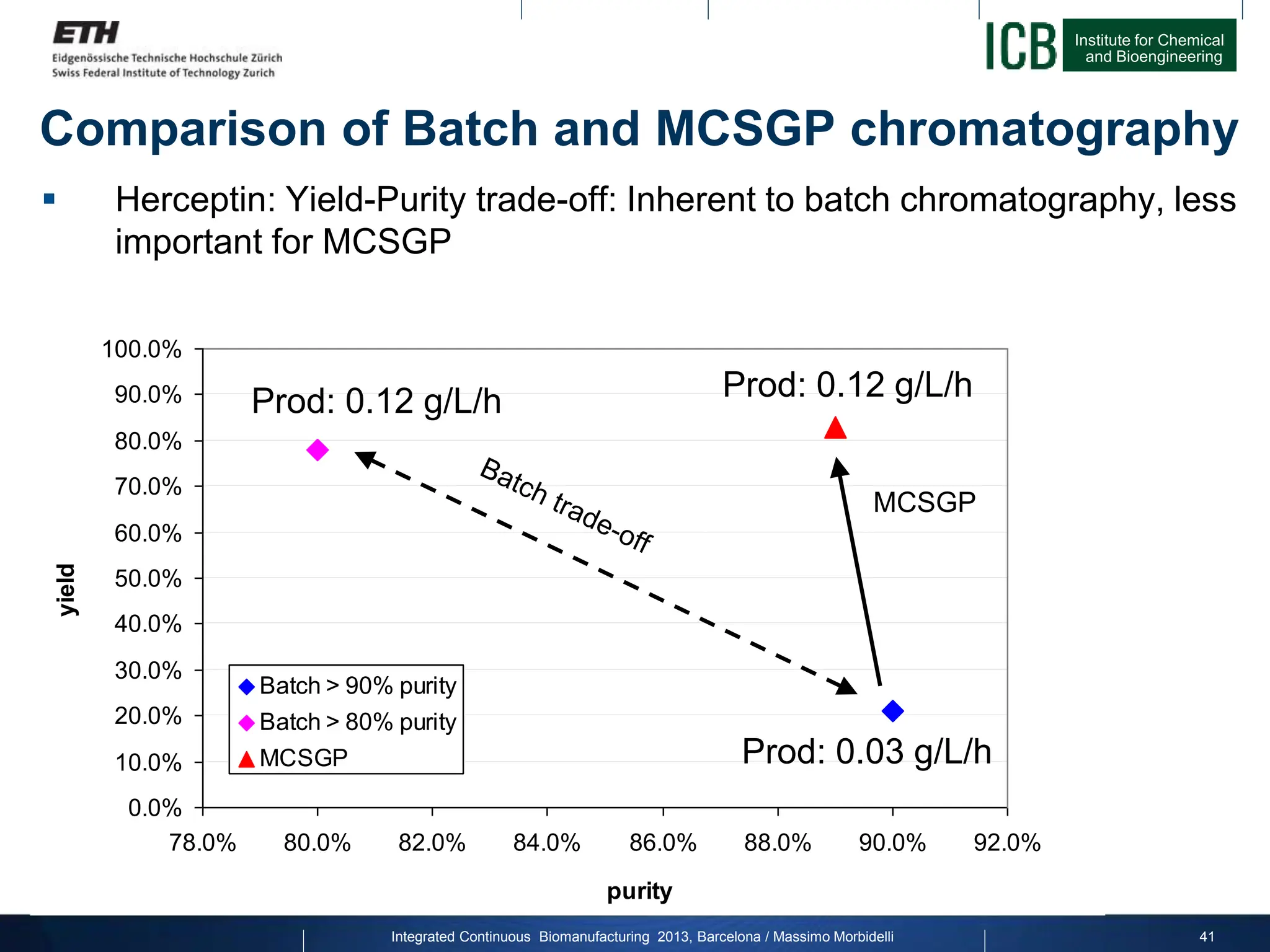
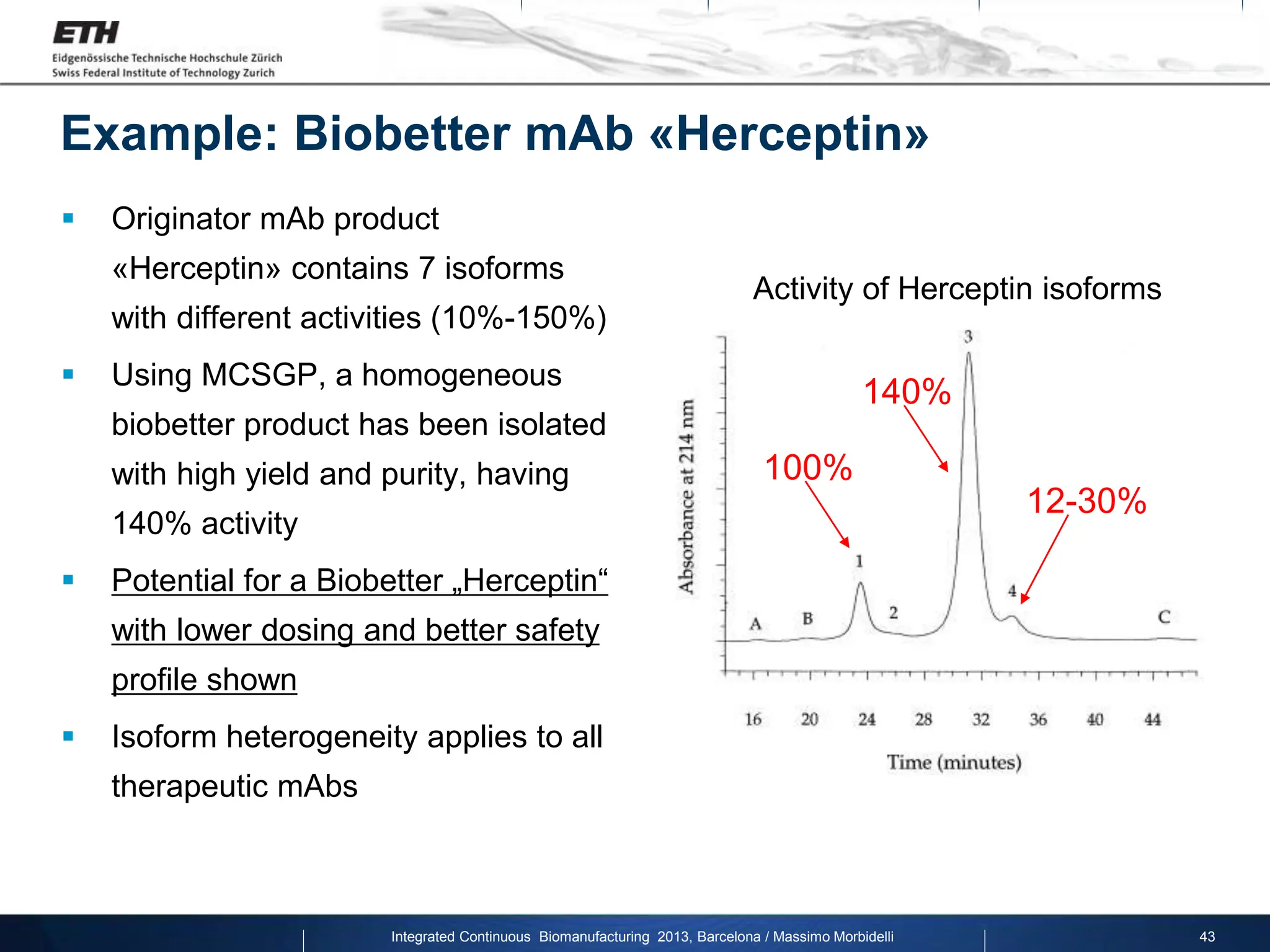
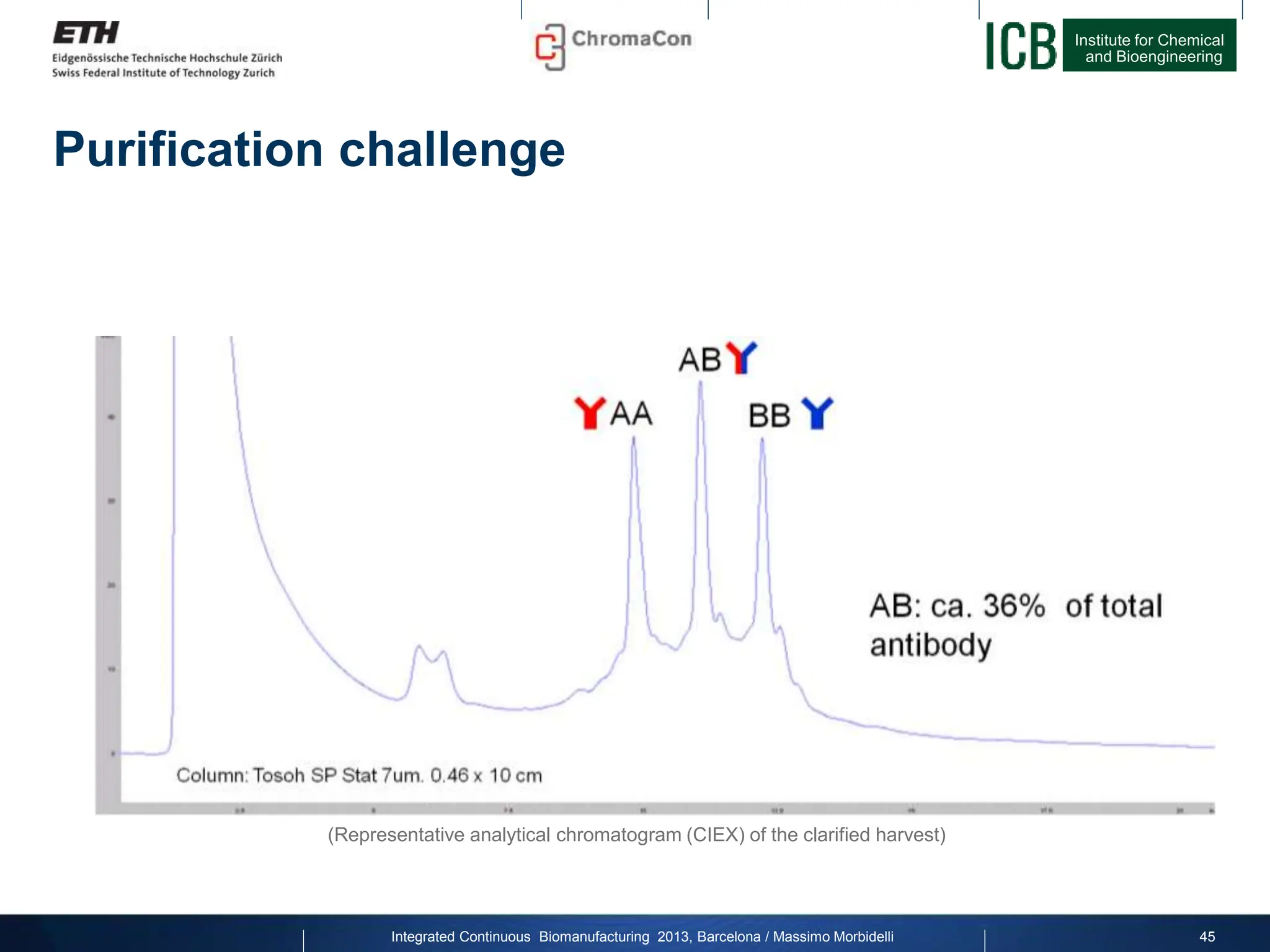
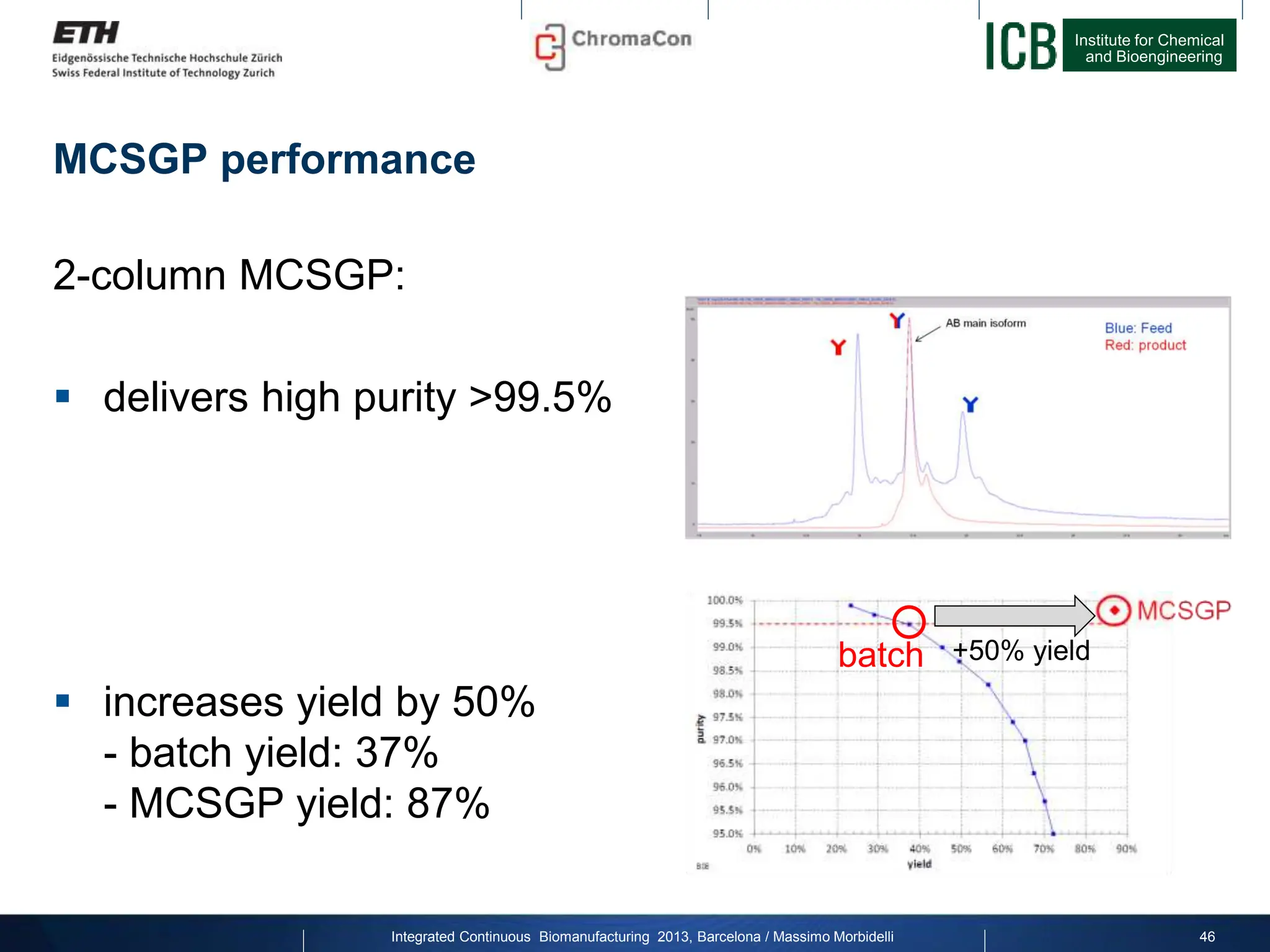
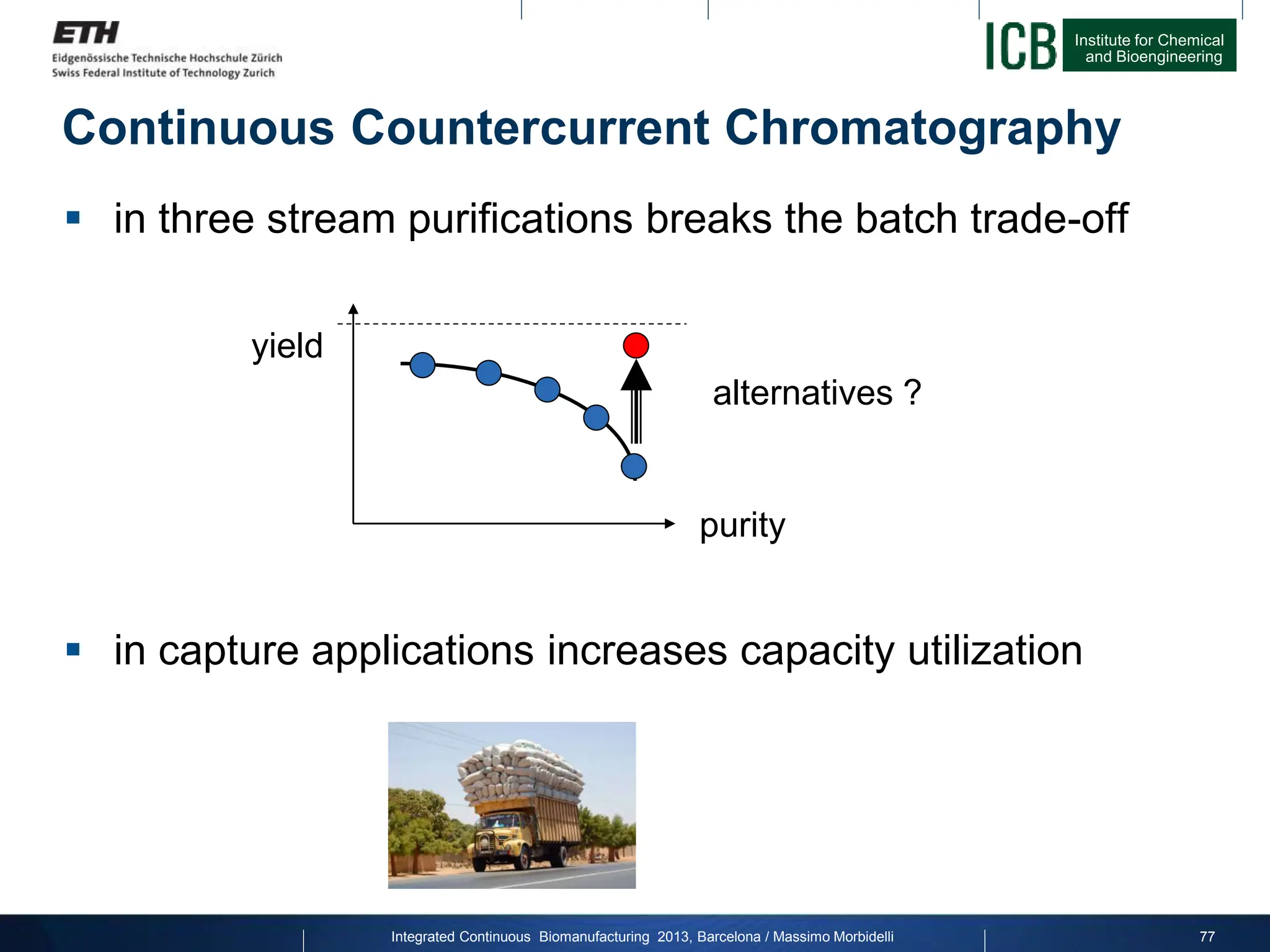
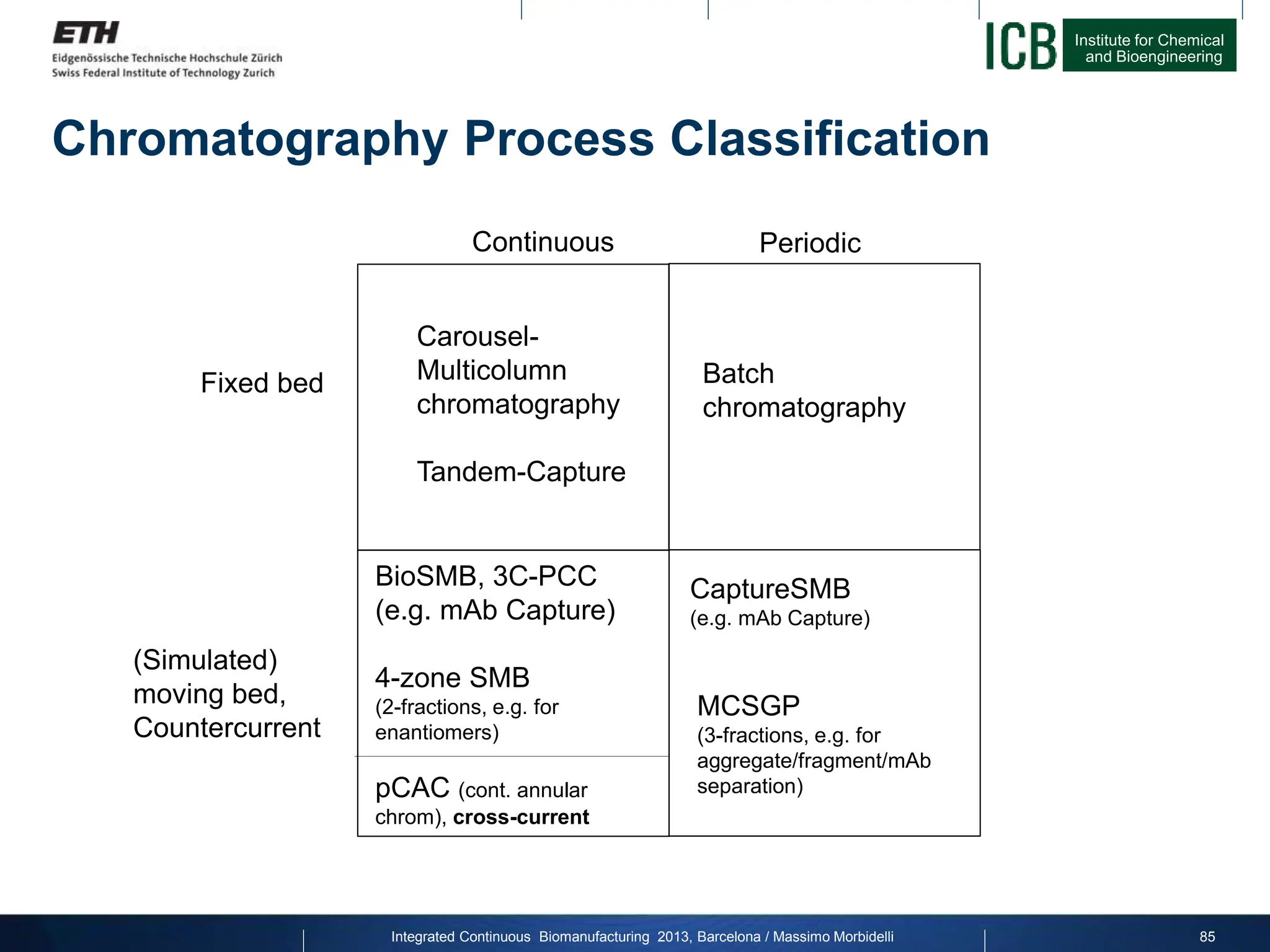
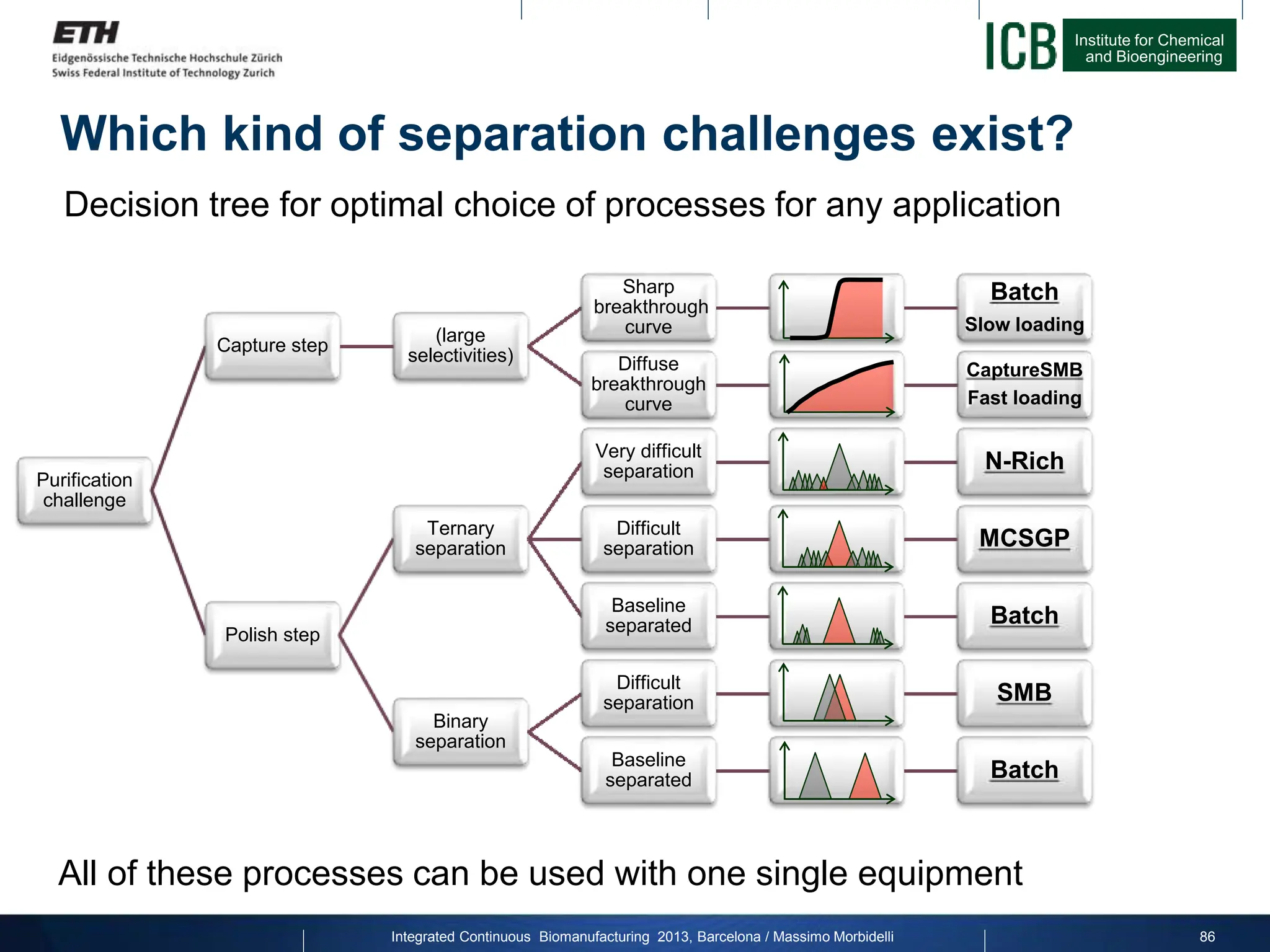
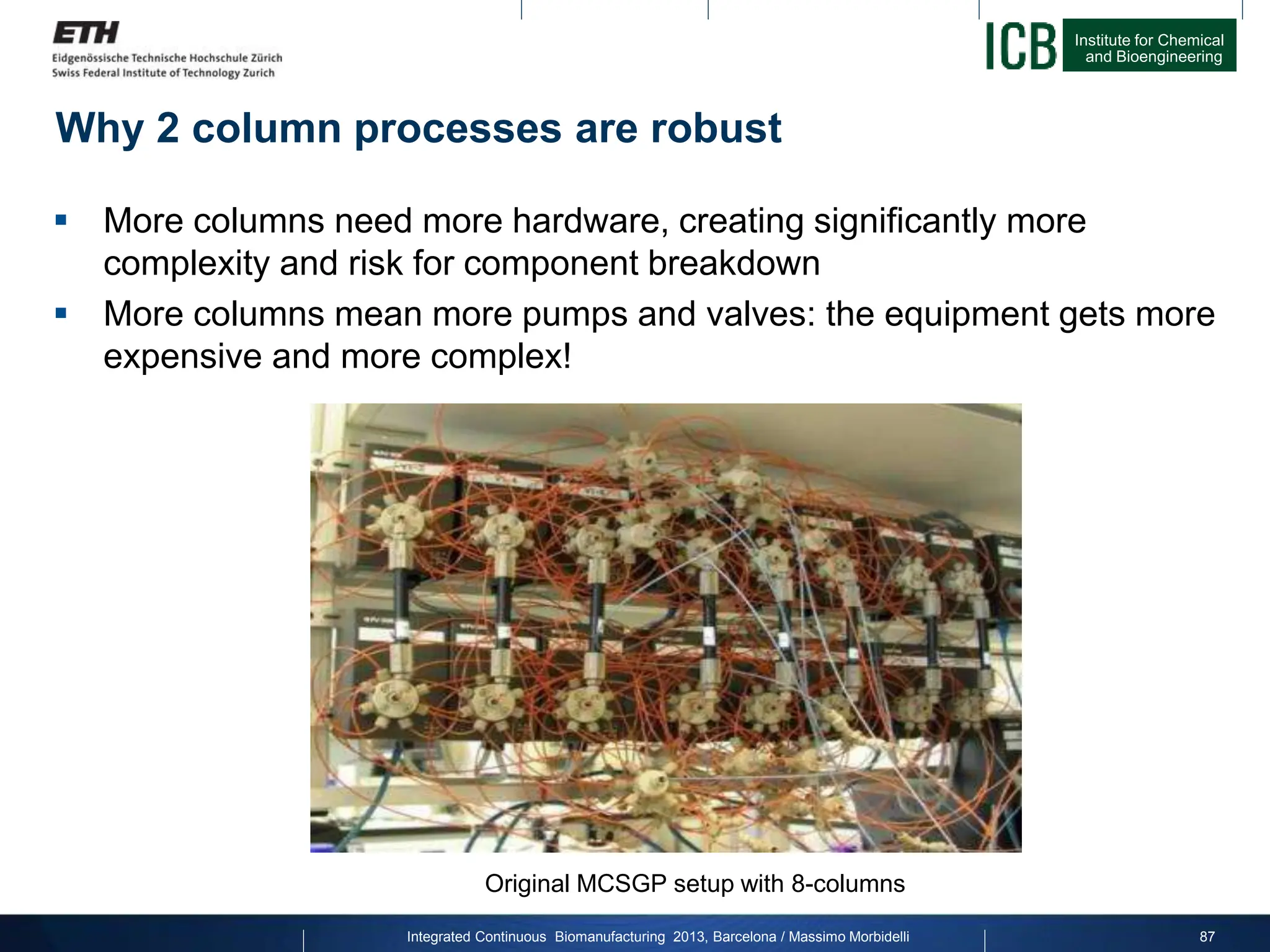
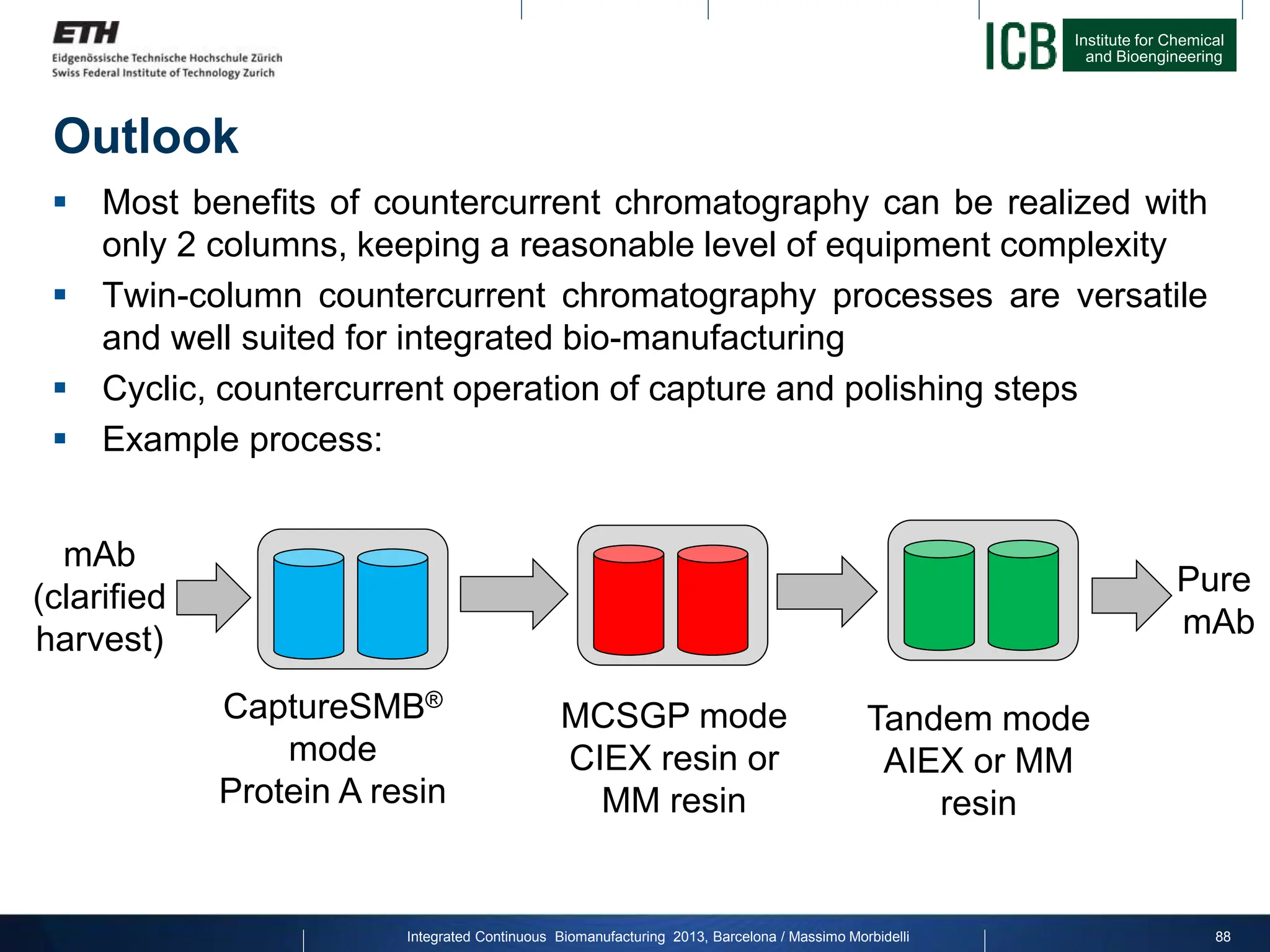
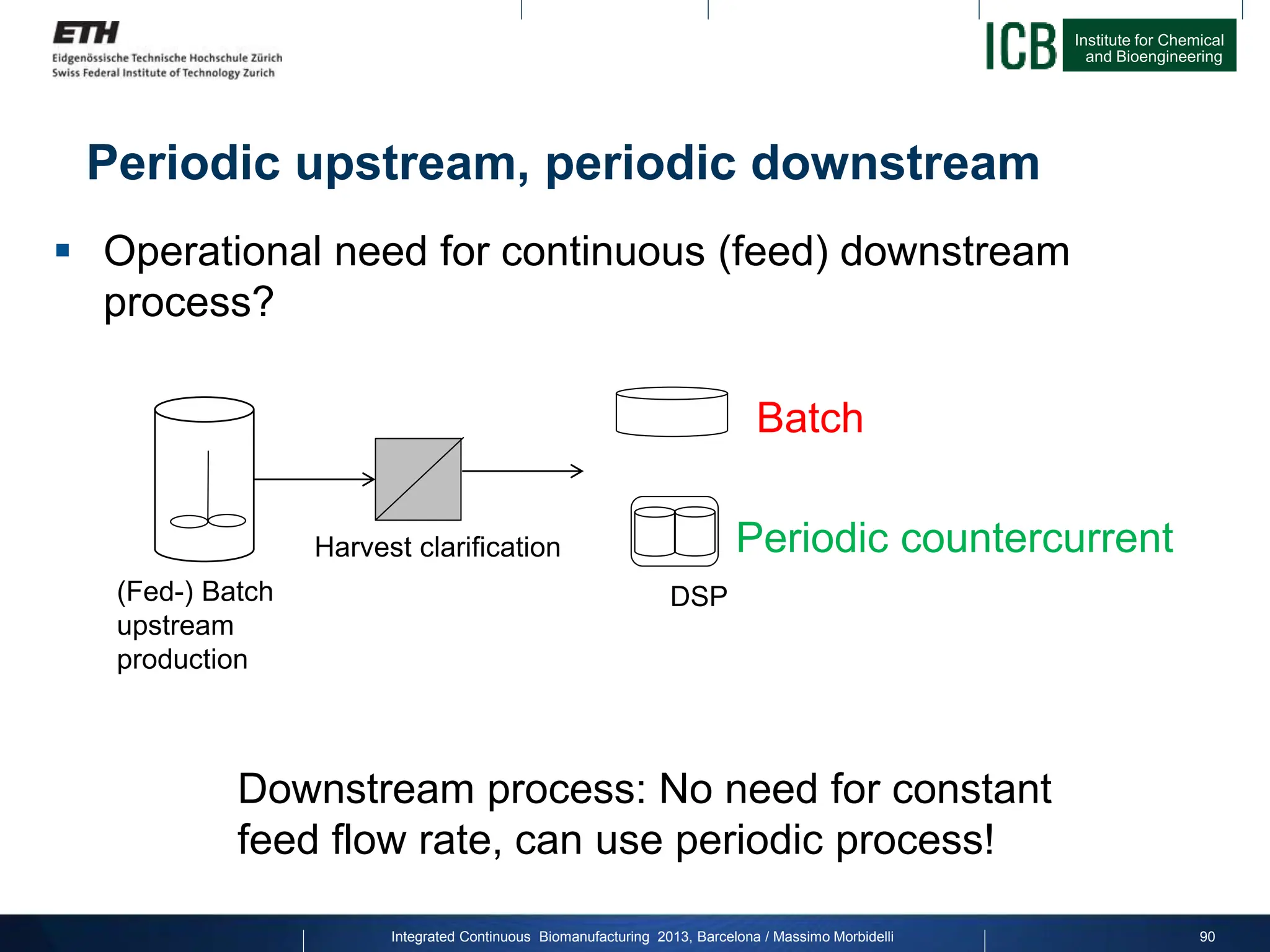
The document discusses multicolumn continuous countercurrent chromatography (MCSGP) as an alternative to batch chromatography for purifying biomolecules like monoclonal antibodies and peptides. MCSGP uses multiple chromatography columns connected in a continuous process to achieve high purity and yield for multi-component separations. It provides examples showing MCSGP can increase productivity 5-25x over batch and improve yield-purity tradeoffs. MCSGP is presented as enabling the production of biobetters and separation of complex molecules like antibody isoforms and bispecific antibodies.

![Institute for Chemical
and Bioengineering
10
Batch versus SMB performance
Separation of a pharmaceutical intermediate racemate
mixture on a chiral stationary phase (CSP)1
1 J.Chrom A 1006 (1-2): 267-280, 2003
0
0.5
1
1.5
2
2.5
3
Solvent requirement Productivity
HPLC Batch
SMB
Eluent need [L/g]
-80%
8x
Productivity [g/ kg/min]
Integrated Continuous Biomanufacturing 2013, Barcelona / Massimo Morbidelli](https://image.slidesharecdn.com/1-morbidelli-131014presentationbarcelonamm-240202071600-e66e82ca/75/1-Morbidelli-131014_Presentation_Barcelona_MM-pptx-6-2048.jpg)






![Institute for Chemical
and Bioengineering
Integrated Continuous Biomanufacturing 2013, Barcelona / Massimo Morbidelli
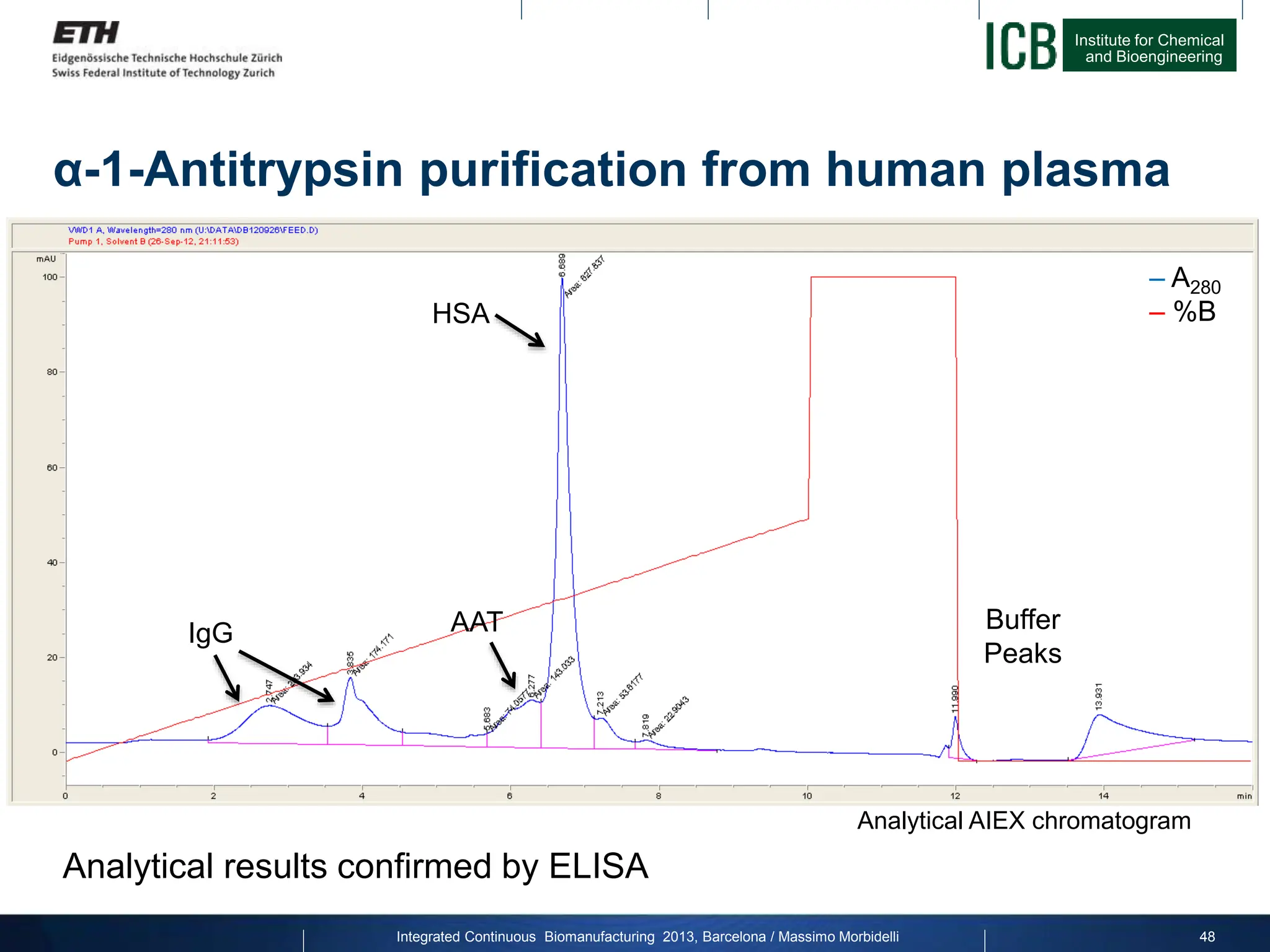
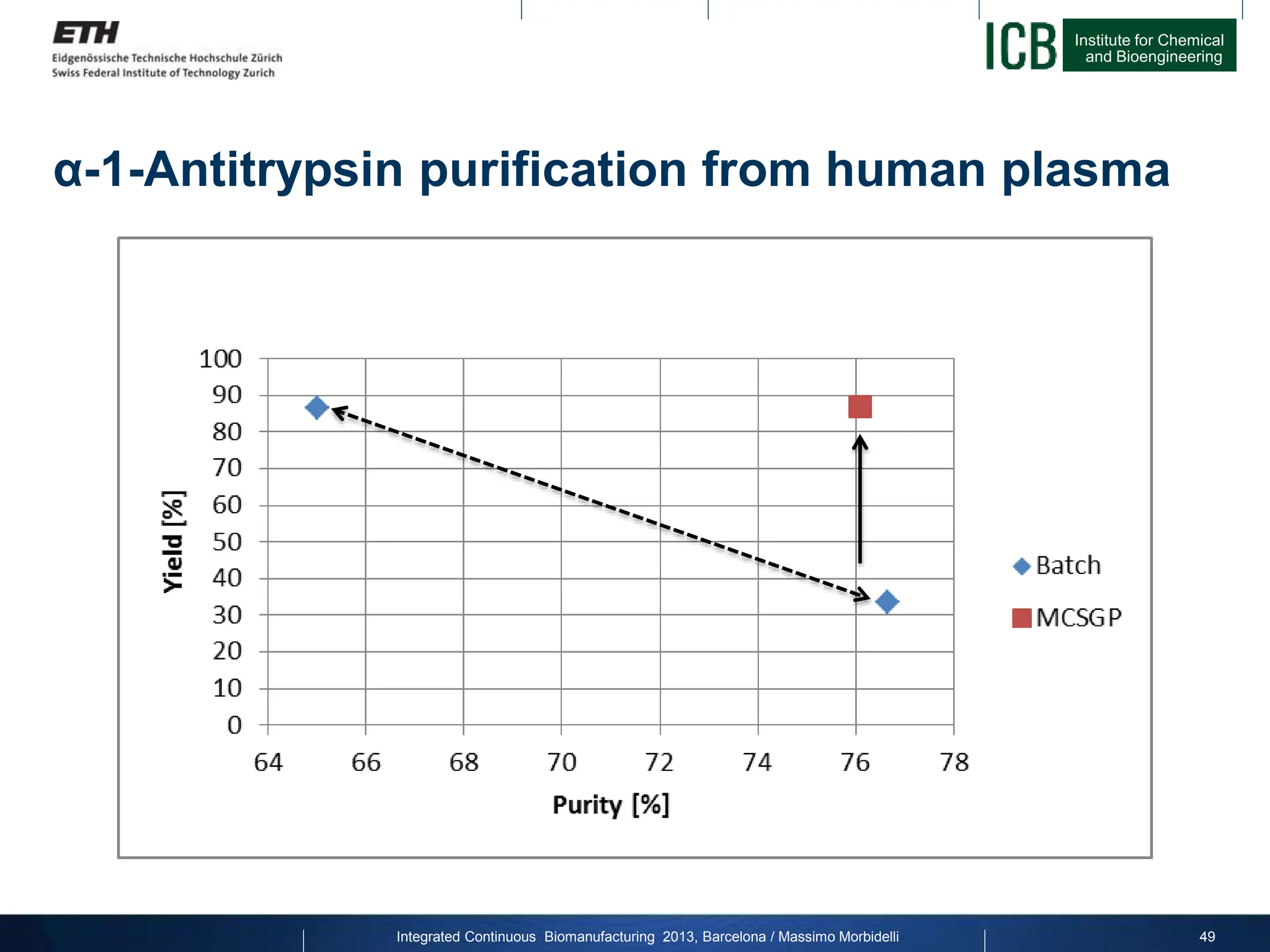
α-1-Antitrypsin purification from human plasma
50
MCSGP
Weak
(IgG, HSA)
Product
(AAT)
Strong
Impurities
Purity [%] Yield [%]
Batch (max. P) 76.66 33.35
Batch (max. Y) 65 86.47
MCSGP 76.08 86.74](https://image.slidesharecdn.com/1-morbidelli-131014presentationbarcelonamm-240202071600-e66e82ca/75/1-Morbidelli-131014_Presentation_Barcelona_MM-pptx-28-2048.jpg)
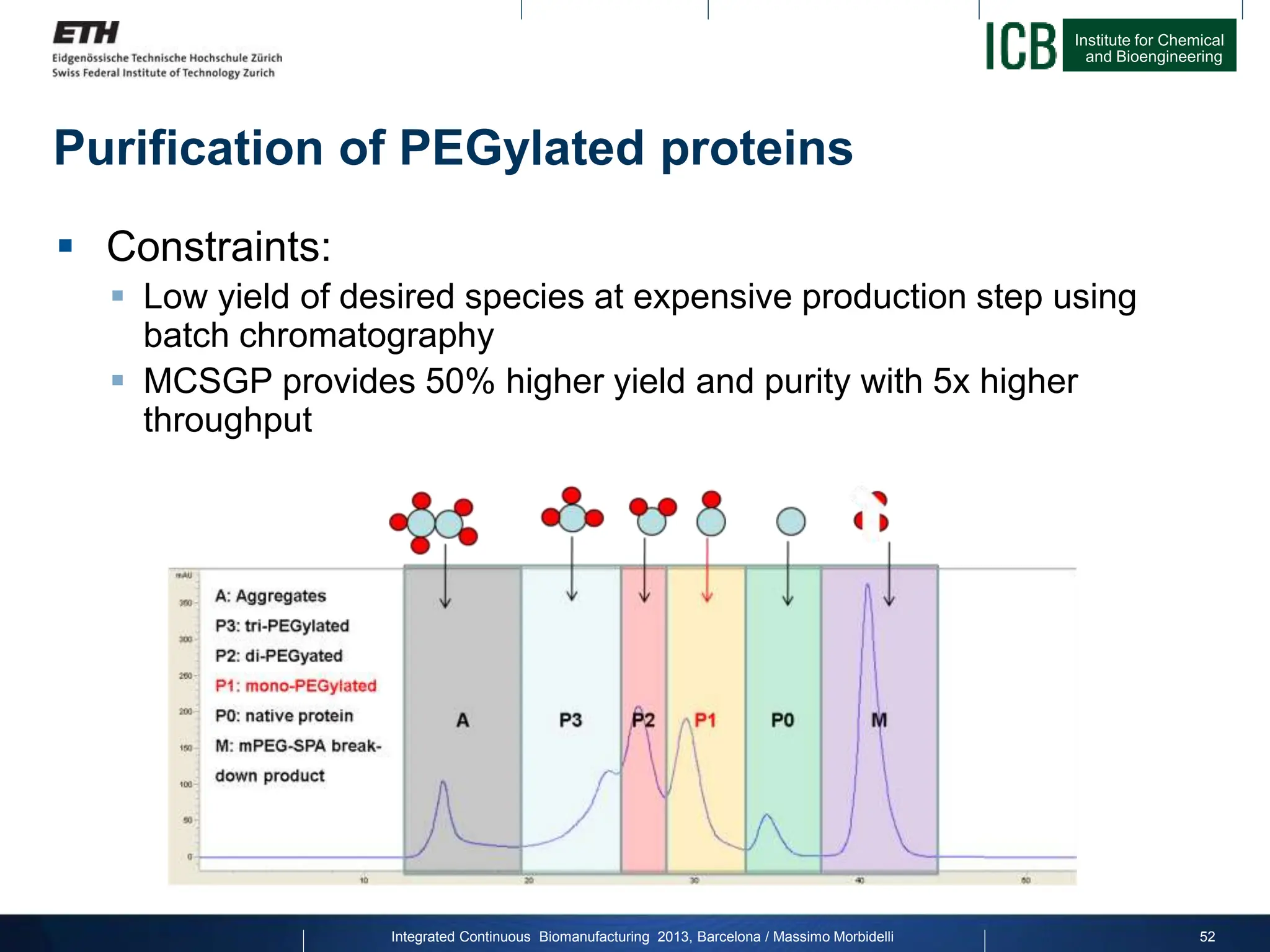
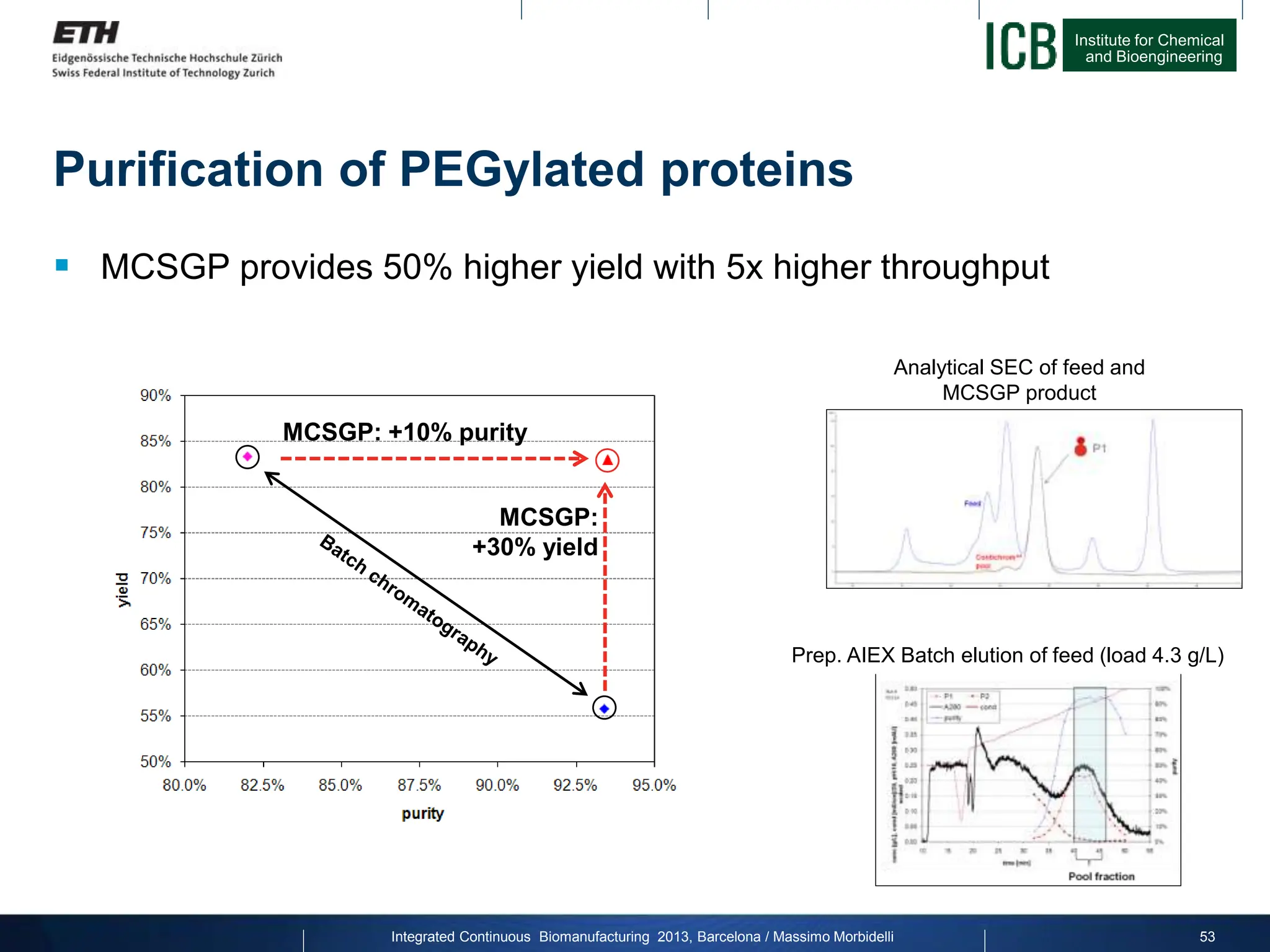

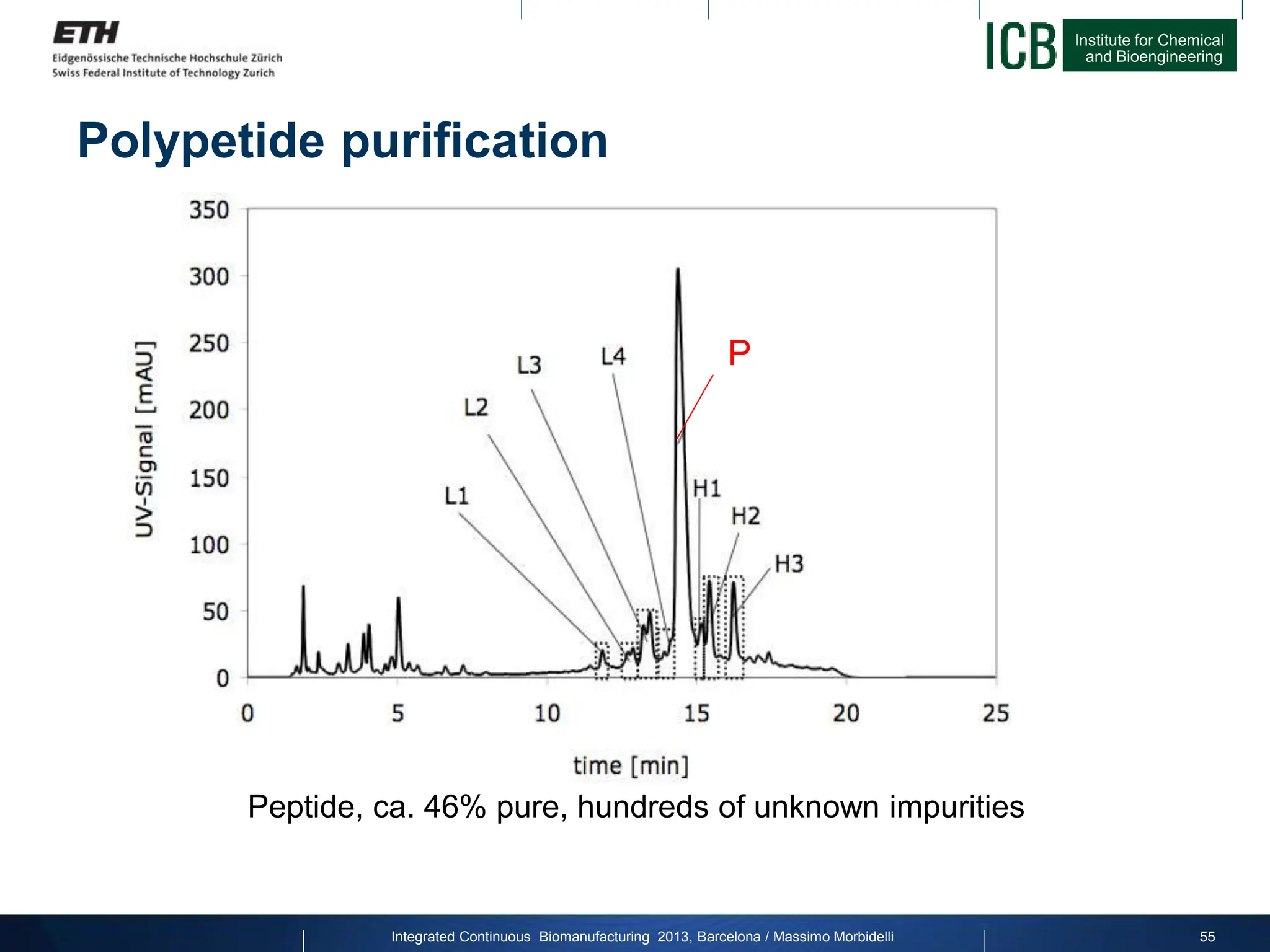
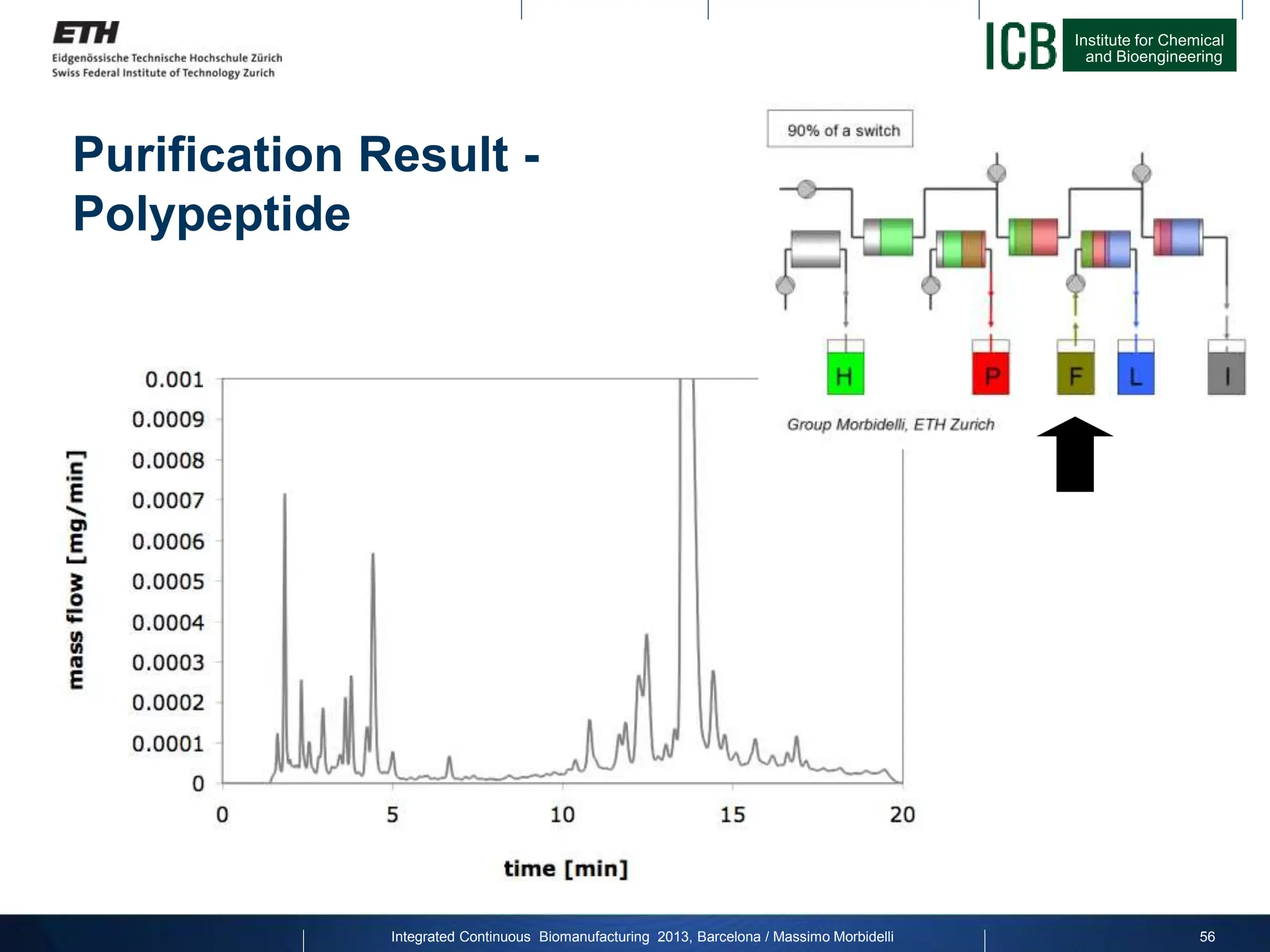
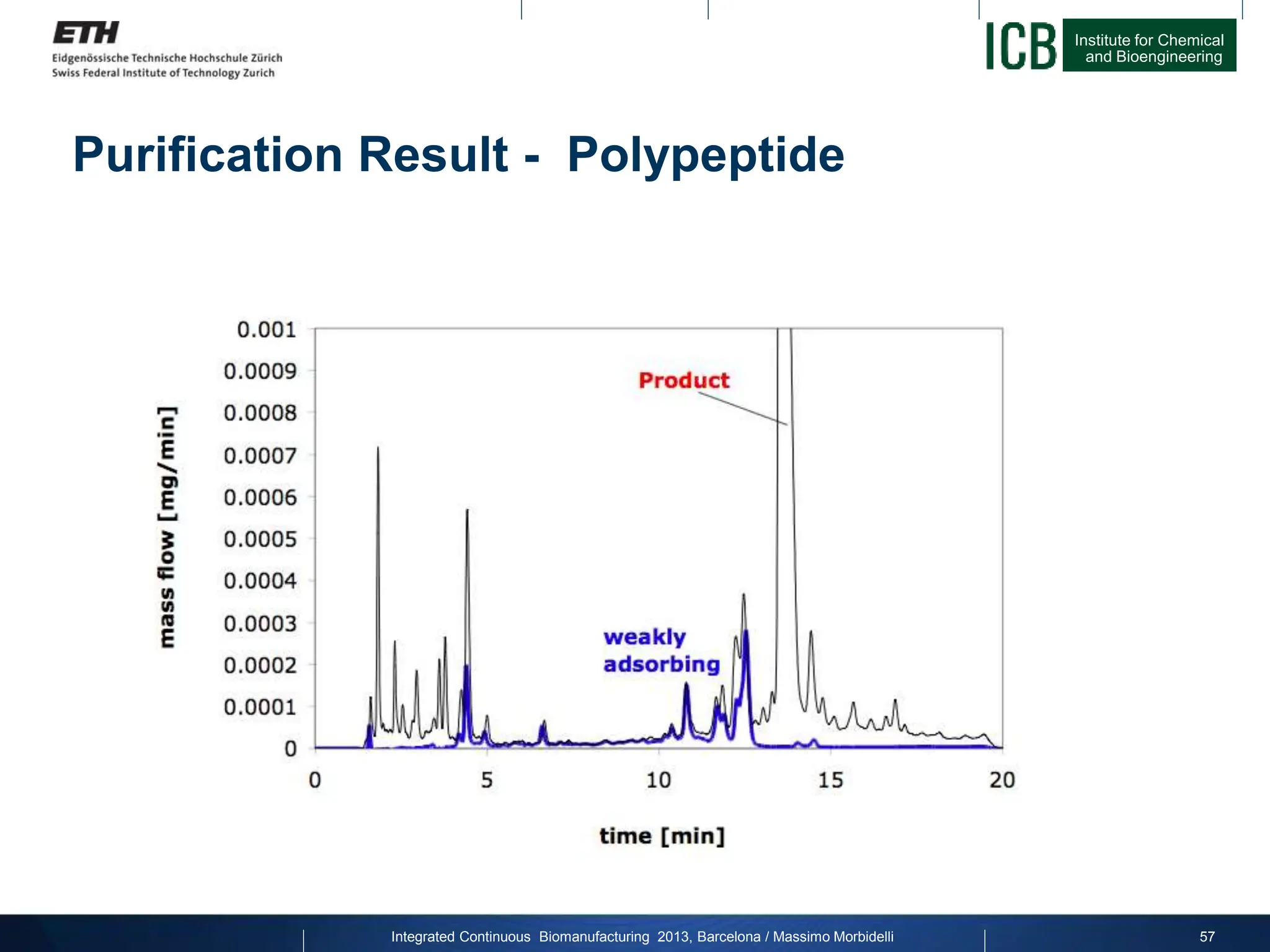
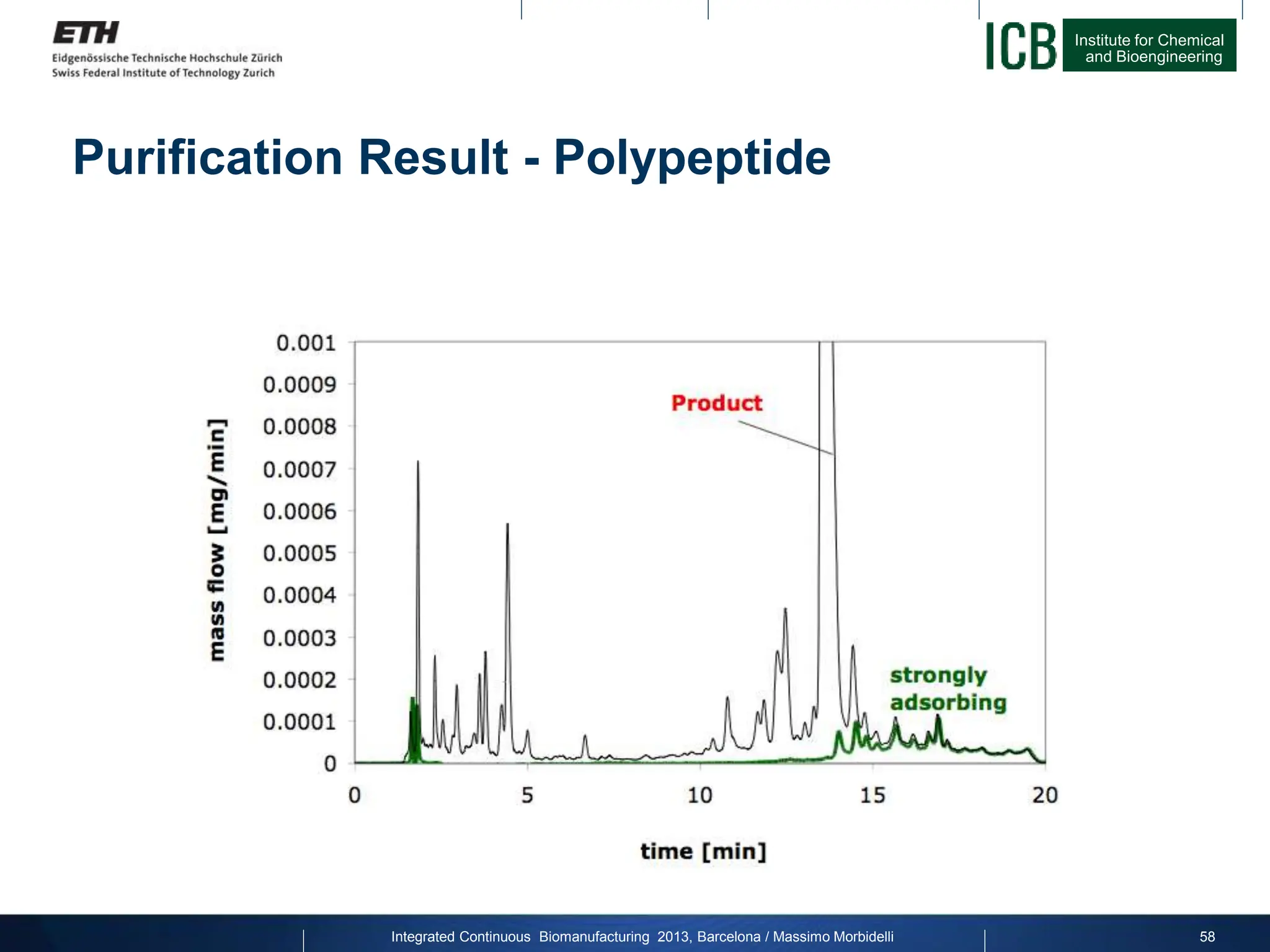
![Institute for Chemical
and Bioengineering
Purification Result - Productivity
factor 25
Joint project with Novartis Pharma on Calcitonin:
Productivity
[g/L/h]
Yield for constant purity [%]
Integrated Continuous Biomanufacturing 2013, Barcelona / Massimo Morbidelli 59](https://image.slidesharecdn.com/1-morbidelli-131014presentationbarcelonamm-240202071600-e66e82ca/75/1-Morbidelli-131014_Presentation_Barcelona_MM-pptx-37-2048.jpg)

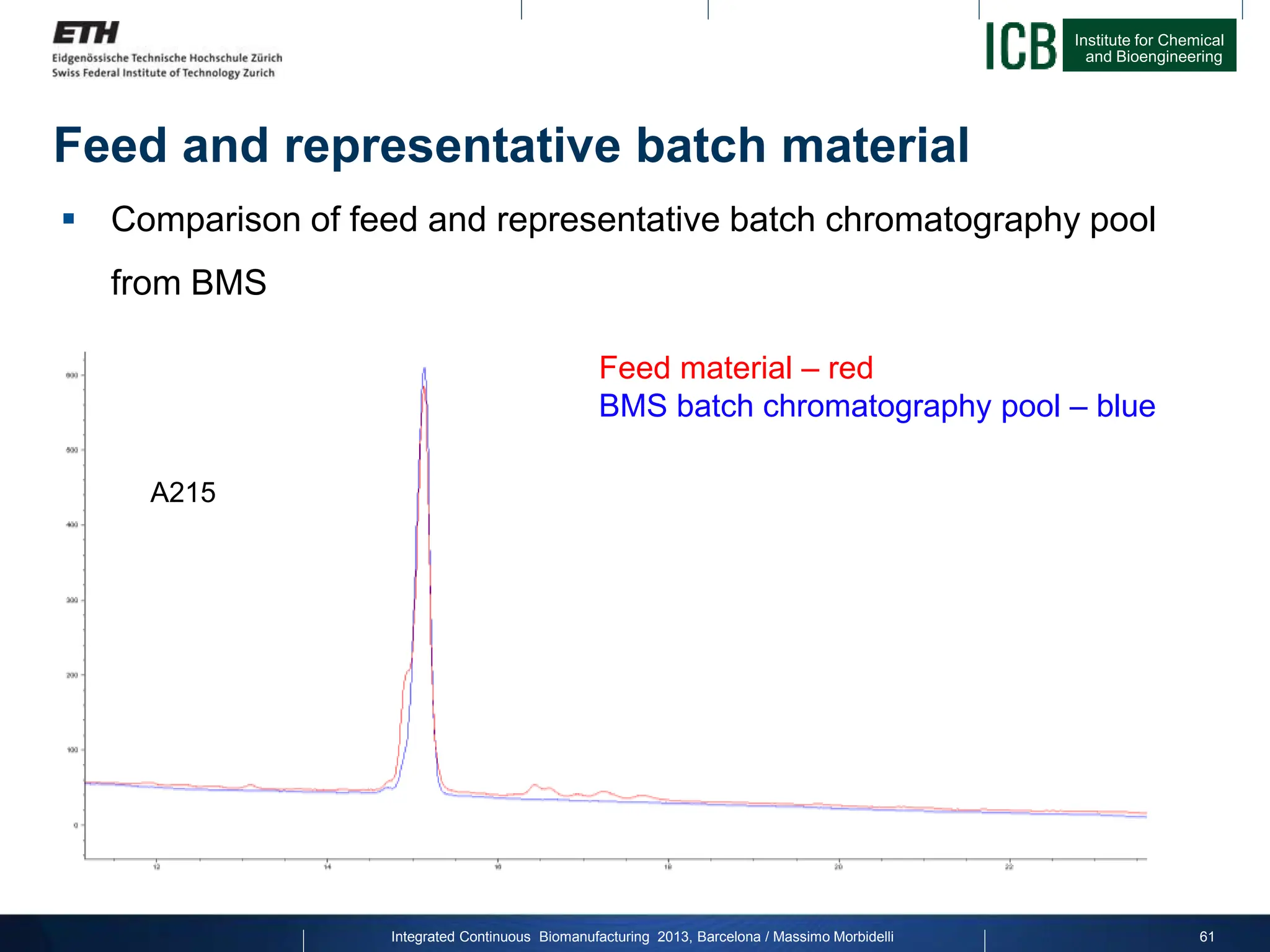
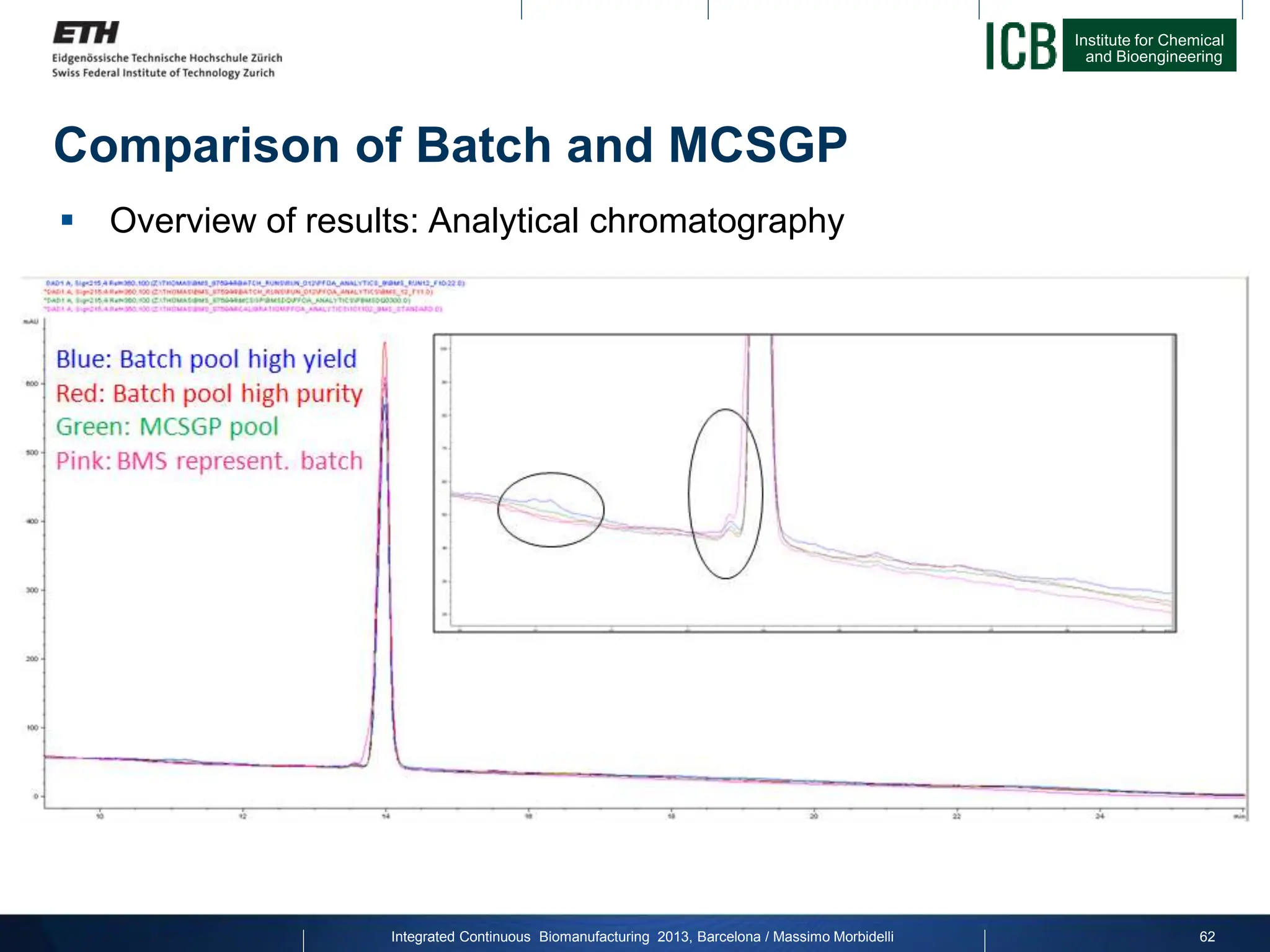
![Institute for Chemical
and Bioengineering
Comparison of Batch and MCSGP
Overview of results:
96.0
96.5
97.0
97.5
98.0
98.5
99.0
0 10 20 30 40 50 60 70 80 90 100
Purity
[%]
Yield [%]
A215
Integrated Continuous Biomanufacturing 2013, Barcelona / Massimo Morbidelli 63](https://image.slidesharecdn.com/1-morbidelli-131014presentationbarcelonamm-240202071600-e66e82ca/75/1-Morbidelli-131014_Presentation_Barcelona_MM-pptx-41-2048.jpg)
![Institute for Chemical
and Bioengineering
Comparison of Batch and MCSGP
Overview of results: Purity-Yield chart.
96.0
96.5
97.0
97.5
98.0
98.5
99.0
0 10 20 30 40 50 60 70 80 90 100
Purity
[%]
Yield [%]
Batch
MCSGP
Prod= 28-31 g/L/h
S.C. =0.9-1.0 L/g
conc.P = 8.4-9.3 g/L
Prod= 14 g/L/h
S.C.=0.7 L/g
conc. P = 3.3 g/L
Prod= 3 g/L/h
S.C.=3.5 L/g
conc. P = 8.2 g/L
Integrated Continuous Biomanufacturing 2013, Barcelona / Massimo Morbidelli 64](https://image.slidesharecdn.com/1-morbidelli-131014presentationbarcelonamm-240202071600-e66e82ca/75/1-Morbidelli-131014_Presentation_Barcelona_MM-pptx-42-2048.jpg)

![Institute for Chemical
and Bioengineering
0
20
40
60
80
100
120
140
160
14 16 18 20 22 24
concentration
(normalized)
Time [min]
Feed
Product
W-fraction
S-fraction
EPA-EE (> 97% pure)
DHA-EE
Impurity
FA-EE
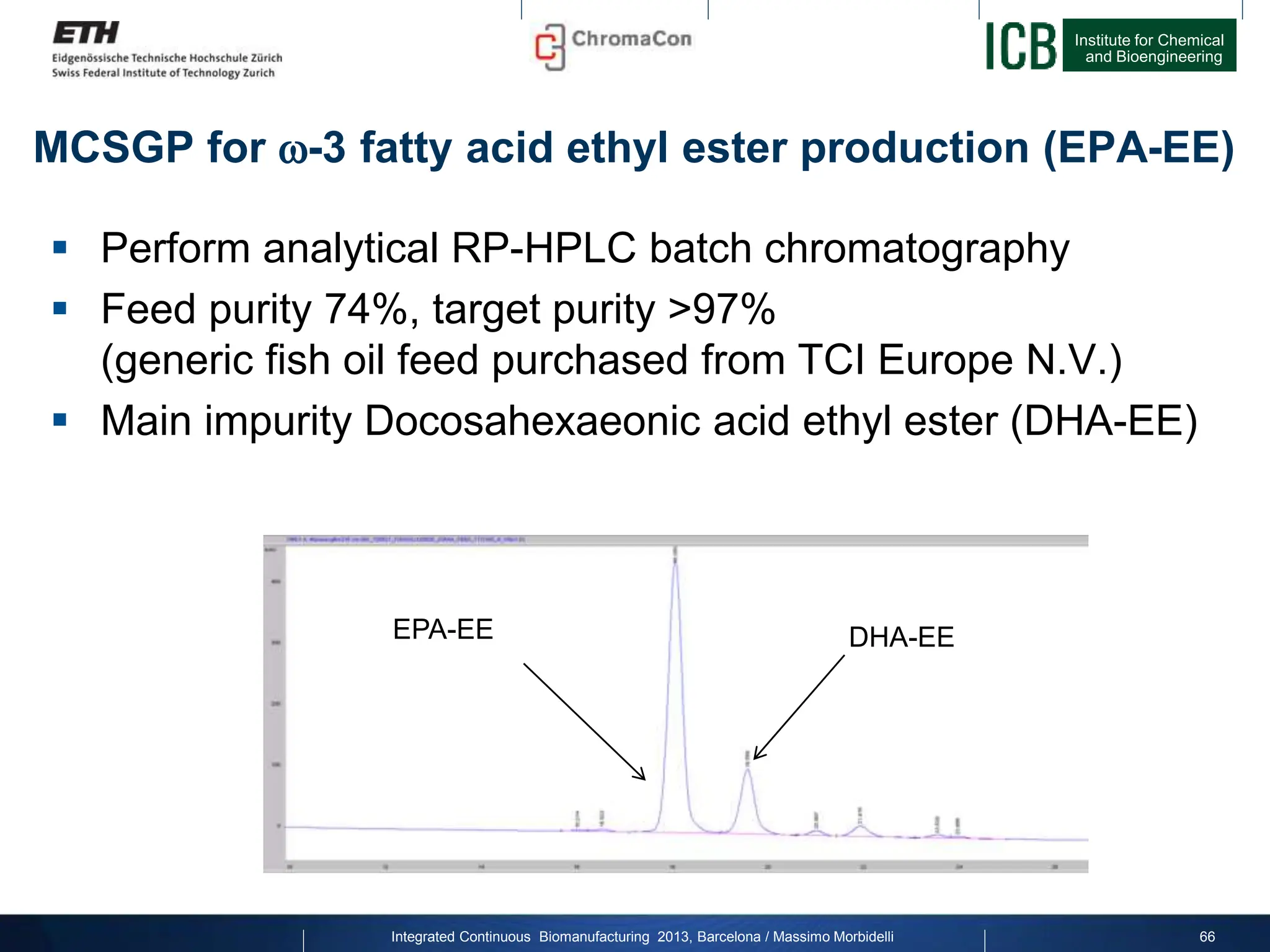
MCSGP for -3 fatty acid ethyl ester production (EPA-EE)
Result chromatograms
69
Integrated Continuous Biomanufacturing 2013, Barcelona / Massimo Morbidelli
Overlay of analytical reversed
phase chromatograms of feed
and fractions from MCSGP
Feed: Ratio EPA/DHA= 4:1](https://image.slidesharecdn.com/1-morbidelli-131014presentationbarcelonamm-240202071600-e66e82ca/75/1-Morbidelli-131014_Presentation_Barcelona_MM-pptx-45-2048.jpg)
![Institute for Chemical
and Bioengineering
MCSGP for -3 fatty acid ethyl ester production (EPA-EE)
Process for production of > 97% purity EPA-EE developed based on
reverse phase chromatography with Ethanol as solvent
Resin & solvent cost reduction of 80% with respect to batch
chromatography
MCSGP
(20 m
resin)
Batch
(15 m
resin)
Improvement by
MCSGP
Purity [%] >97% >97%
Yield [%] 90% 36% + 250%
Productivity (Throughput)
[(g product)/(L resin)/(hr operation time)]
65 11 + 590%
Solvent Consumption
[L solvent/g product]
0.8 3.2 - 75%
Integrated Continuous Biomanufacturing 2013, Barcelona / Massimo Morbidelli 70](https://image.slidesharecdn.com/1-morbidelli-131014presentationbarcelonamm-240202071600-e66e82ca/75/1-Morbidelli-131014_Presentation_Barcelona_MM-pptx-46-2048.jpg)

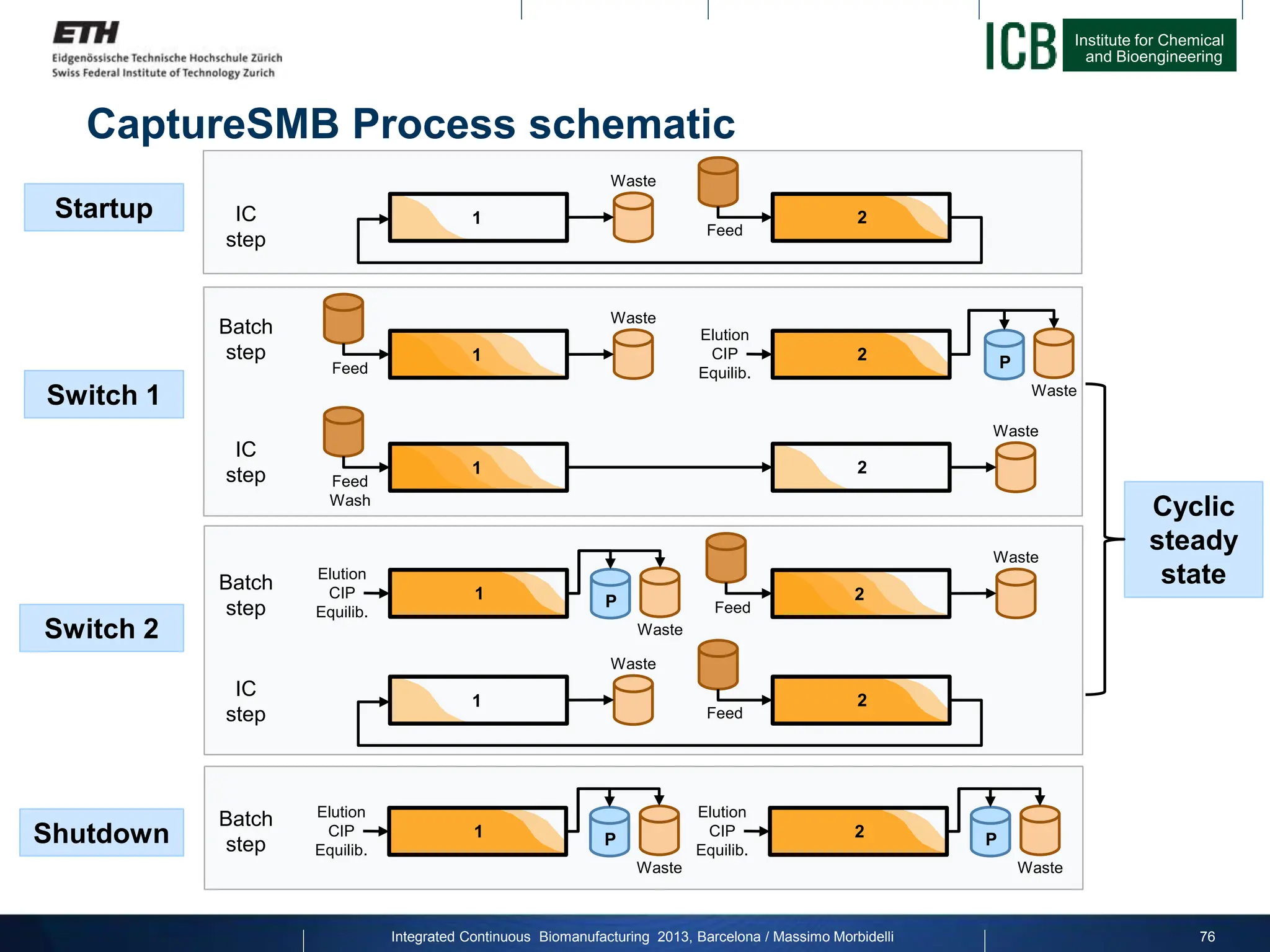



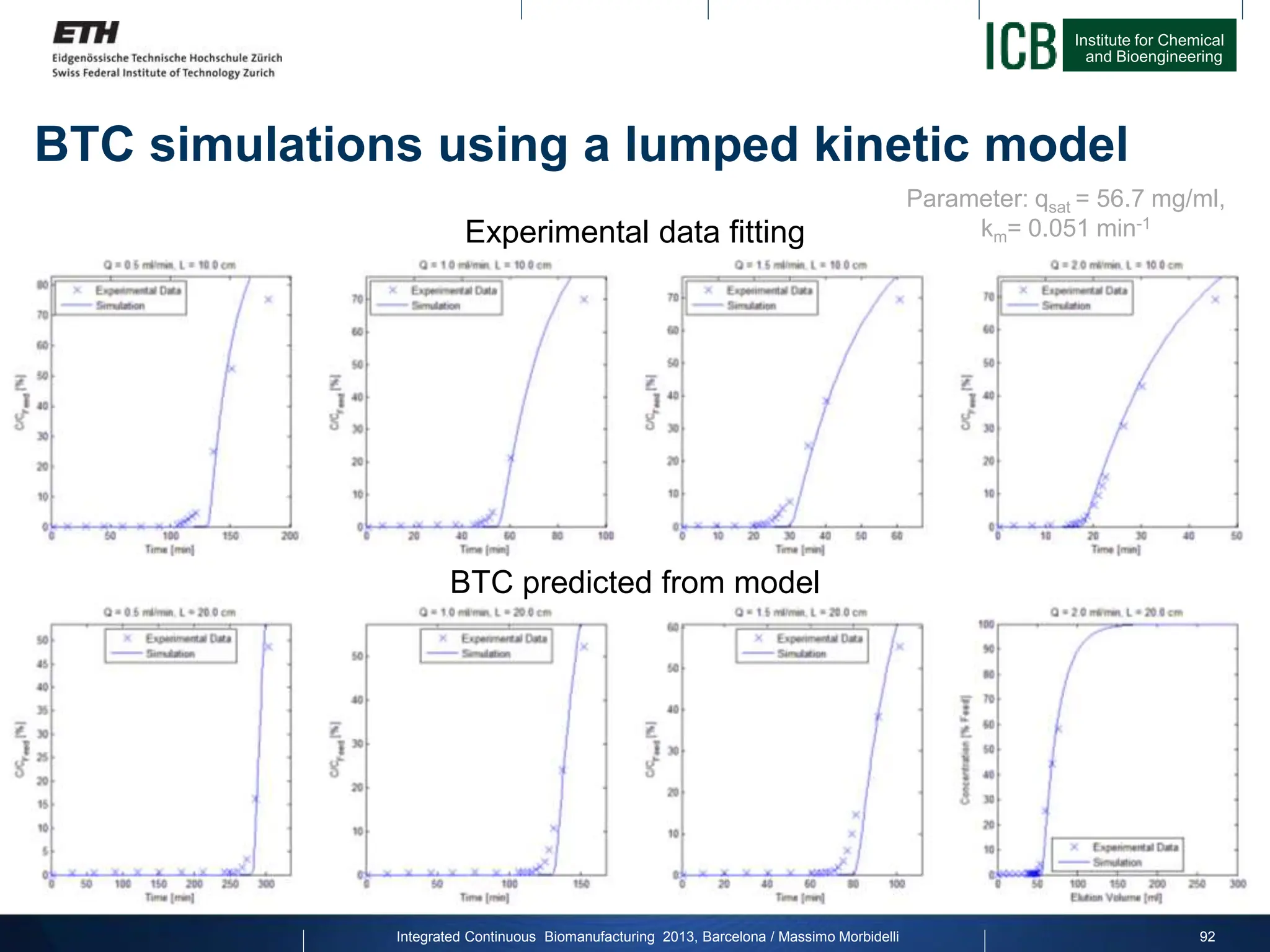
![Institute for Chemical
and Bioengineering
Buffers:
Method:
Experimental conditions: Batch chromatography
Equilibration A 20 mM Phos, 150 mM NaCl, pH 7.5
Wash B 20 mM Phos, 1 M NaCl, pH 7.5
Elution C 50 mM Na-Cit, pH 3.2
CIP D 0.1 M NaOH
93
Step CV [ml]
Equilibration (A) 5
Load
Wash-1 (A) 5
Wash-2 (B) 5
Wash-3 (A) 5
Elution (C) 5
CIP (D) 7.5
Re-Equi-1 (C) 2
Re-Equi-2 (A) 3
Integrated Continuous Biomanufacturing 2013, Barcelona / Massimo Morbidelli](https://image.slidesharecdn.com/1-morbidelli-131014presentationbarcelonamm-240202071600-e66e82ca/75/1-Morbidelli-131014_Presentation_Barcelona_MM-pptx-64-2048.jpg)
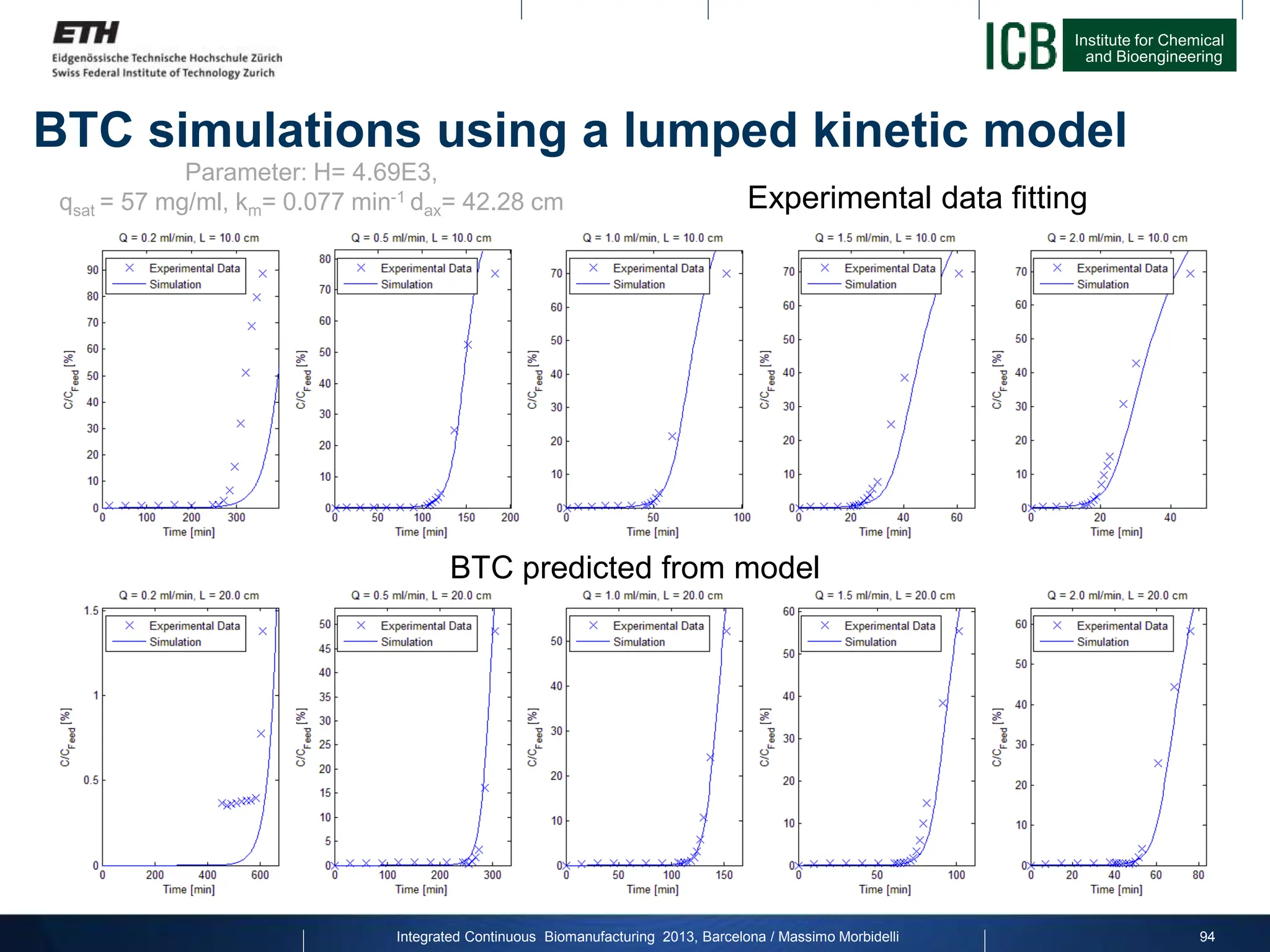
![Institute for Chemical
and Bioengineering
Integrated Continuous Biomanufacturing 2013, Barcelona / Massimo Morbidelli
Internal concentration profiles: 3-Col process
95
2 4 6 8 10
0
1
2
c
[mg/ml]
Column 1: Regenerating
2 4 6 8 10
0
20
40
60
80
Column Position [cm]
q
[mg/ml]
2 4 6 8 10
0
1
2
Column 2: Loading
2 4 6 8 10
0
20
40
60
80
Column Position [cm]
2 4 6 8 10
0
1
2
Column 3: FT uptake
2 4 6 8 10
0
20
40
60
80
Column Position [cm]
Simulation parameters: lumped kinetic model
Q= 0.84 ml/min, H= 4.69E3, qsat = 55 mg/ml, km= 0.077 min-1](https://image.slidesharecdn.com/1-morbidelli-131014presentationbarcelonamm-240202071600-e66e82ca/75/1-Morbidelli-131014_Presentation_Barcelona_MM-pptx-66-2048.jpg)
![Institute for Chemical
and Bioengineering
Economic evaluation: buffer consumption per year
96
Significant buffer consumption
savings achieved using
Amsphere JWT 203 and
CaptureSMB
PoC Phase III Commercial
Product per harvest [kg] 4 10 24
Fermenter harvest size [L] 2000 5000 12000
Product concentration [g/L] 2 2 2
Harvests per year [-] 8 8 8
Effective production per year [Kg] 32 80 192
Harvest processing time [h] 24 24 24
Resin lifetime [-] 1 harvest 4 harvests 200 cycles
Resin exchange after max. [Year] n.a. n.a. 1
Resin costs AmsphereTM
[US$/L] 13000 13000 13000
Resin costs Agarose [US$/L] 17500 17500 17500
0
50
100
150
200
250
PoC Ph III Comm.
[1000
L]
Buffer consumption per year
(300 cm/h)
0
50
100
150
200
250
PoC Ph III Comm.
[1000
L]
Buffer consumption per year
(600 cm/h)
Integrated Continuous Biomanufacturing 2013, Barcelona / Massimo Morbidelli](https://image.slidesharecdn.com/1-morbidelli-131014presentationbarcelonamm-240202071600-e66e82ca/75/1-Morbidelli-131014_Presentation_Barcelona_MM-pptx-67-2048.jpg)