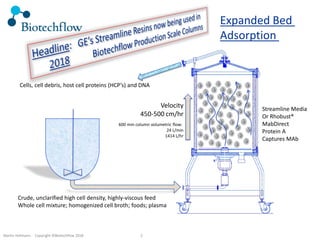
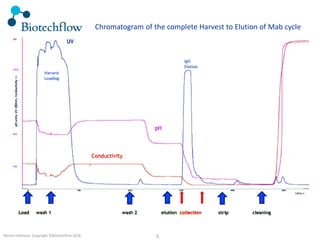
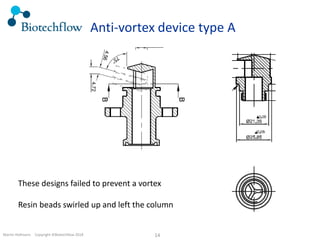
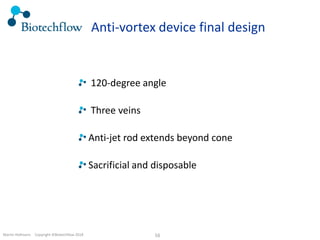
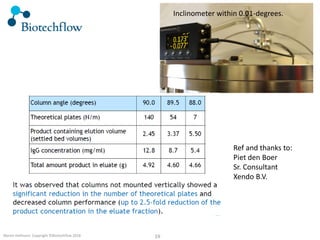
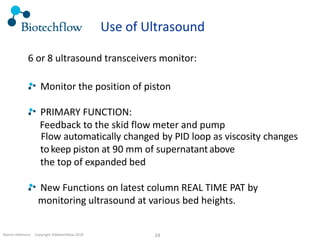
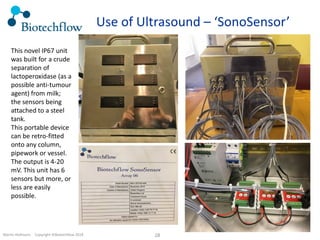
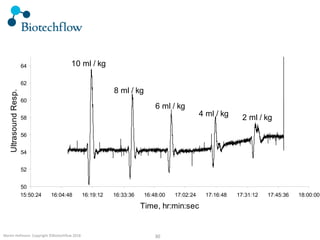
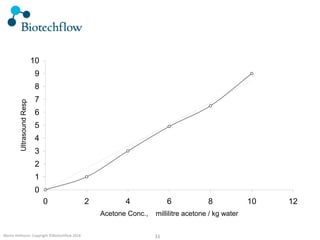
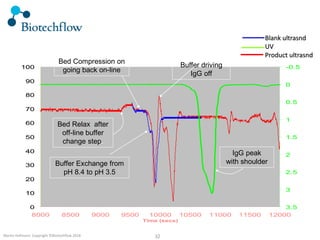
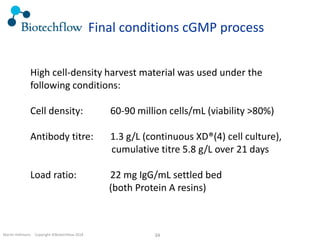
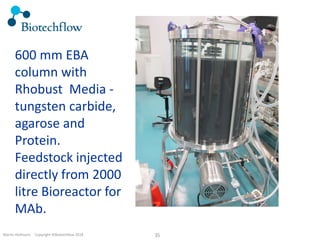
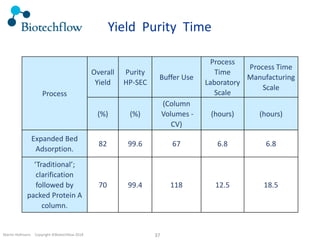
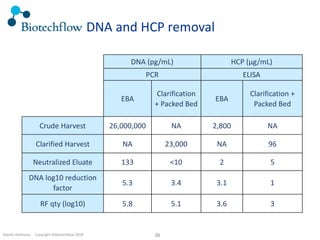
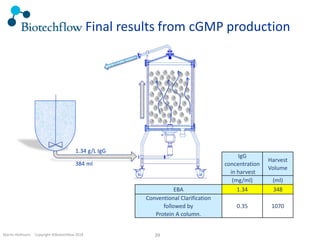
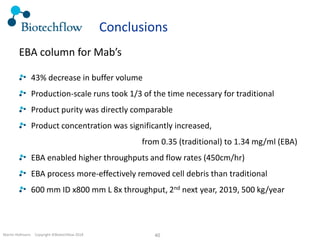
The document outlines the commercial purification of monoclonal antibodies (mAbs) using expanded bed chromatography (EBA) with real-time process analytical technology (PAT) control via ultrasound. It details the operational conditions, design, and effectiveness of the method, emphasizing improved yield and purity compared to traditional methods. Results indicate a significant enhancement in mAb concentration and a reduction in production time and buffer volume usage.