Embed presentation
Download to read offline
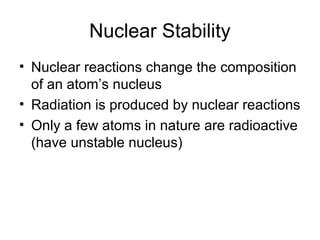
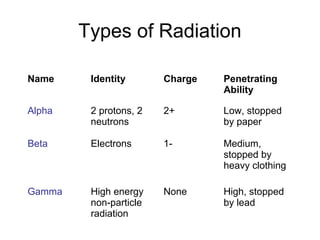


Nuclear reactions can change the composition of an atom's nucleus, producing different types of radiation. Only some atoms are radioactive due to having unstable nuclei. There are three main types of radiation - alpha particles, beta particles, and gamma rays - which differ in their electric charge and ability to penetrate matter, with gamma rays being the most penetrating. Radioactive decay occurs when an unstable nucleus decomposes and emits radiation to form a more stable nucleus, which can be represented through nuclear equations such as the alpha decay of gold-185 producing iridium-181 and four alpha particles.



