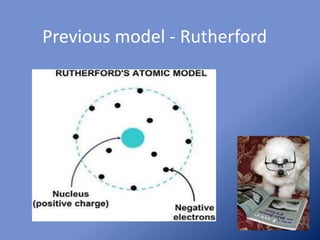
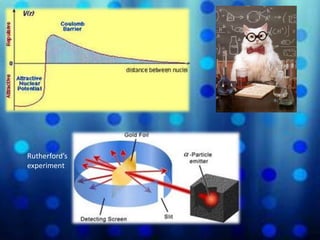
Chadwick discovered a new particle in the nucleus called the neutron through experiments bombarding various elements with alpha particles. The neutron was predicted by Majorana due to its lack of an electrical charge, allowing it to penetrate nuclei without overcoming electromagnetic repulsion like protons. Using Rutherford's zinc shield technique, Chadwick determined neutrons could enter even the heaviest element nuclei, helping establish the neutron as a key part of the nuclear model and defining the atom's mass.