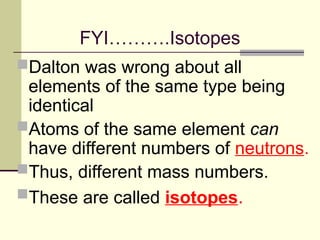
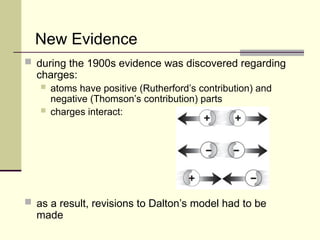
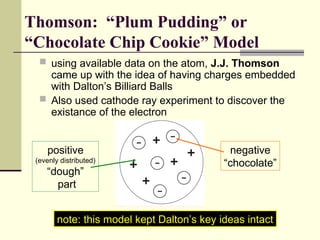
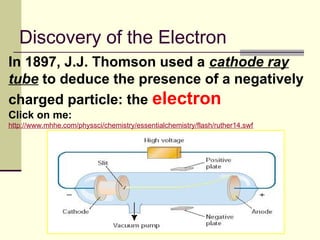
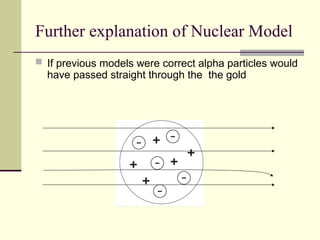
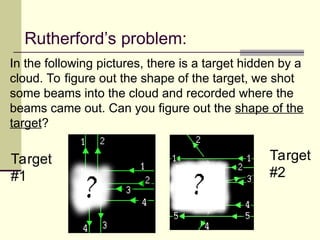
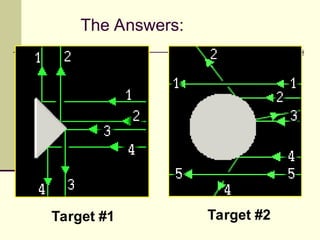
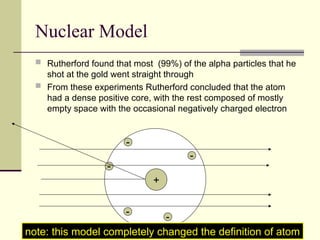
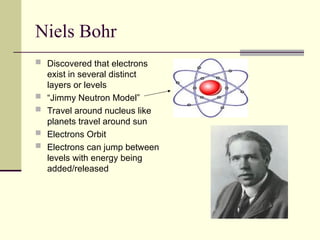
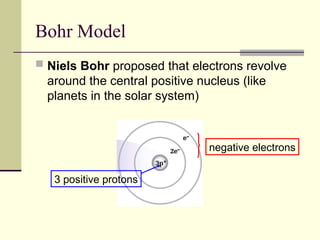
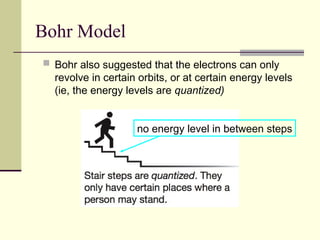
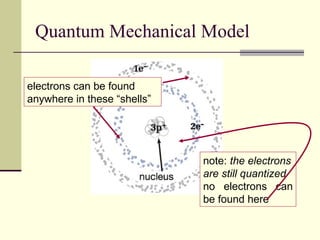
The document outlines the historical development of atomic theory, starting from Democritus's concept of atoms to Dalton's atomic theory and the introduction of isotopes by Frederick Soddy. It explains key experiments and models including Thomson's 'plum pudding' model, Rutherford's nuclear model, and Bohr's model of electrons. The latest understanding of atoms is presented through the quantum mechanical model, emphasizing the complex nature of electron behavior.