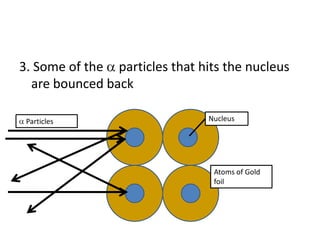
1. Rutherford continued Lenard's experiment by firing positively charged particles at a gold foil.
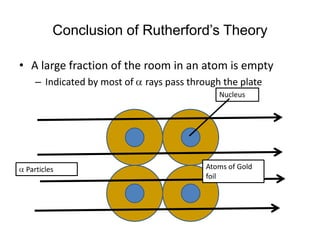
2. He discovered that most particles passed through the foil, but some were deflected or bounced back, indicating a small, dense nucleus at the atom's center.
3. This led Rutherford to propose his nuclear model of the atom, where electrons orbit a tiny, massive nucleus - overturning Thomson's "plum pudding" model. However, his theory did not explain how electrons can orbit the nucleus without radiating energy.