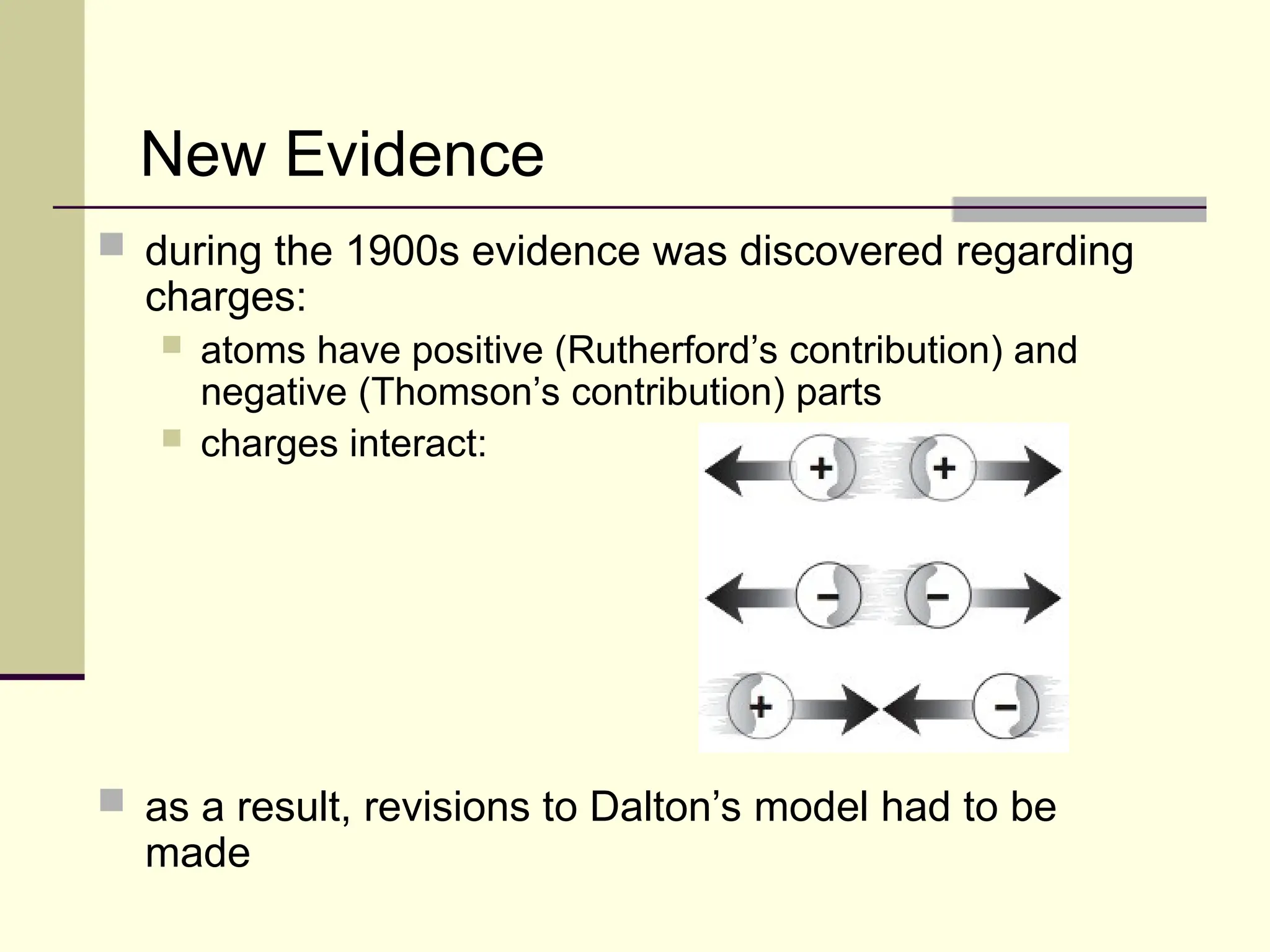
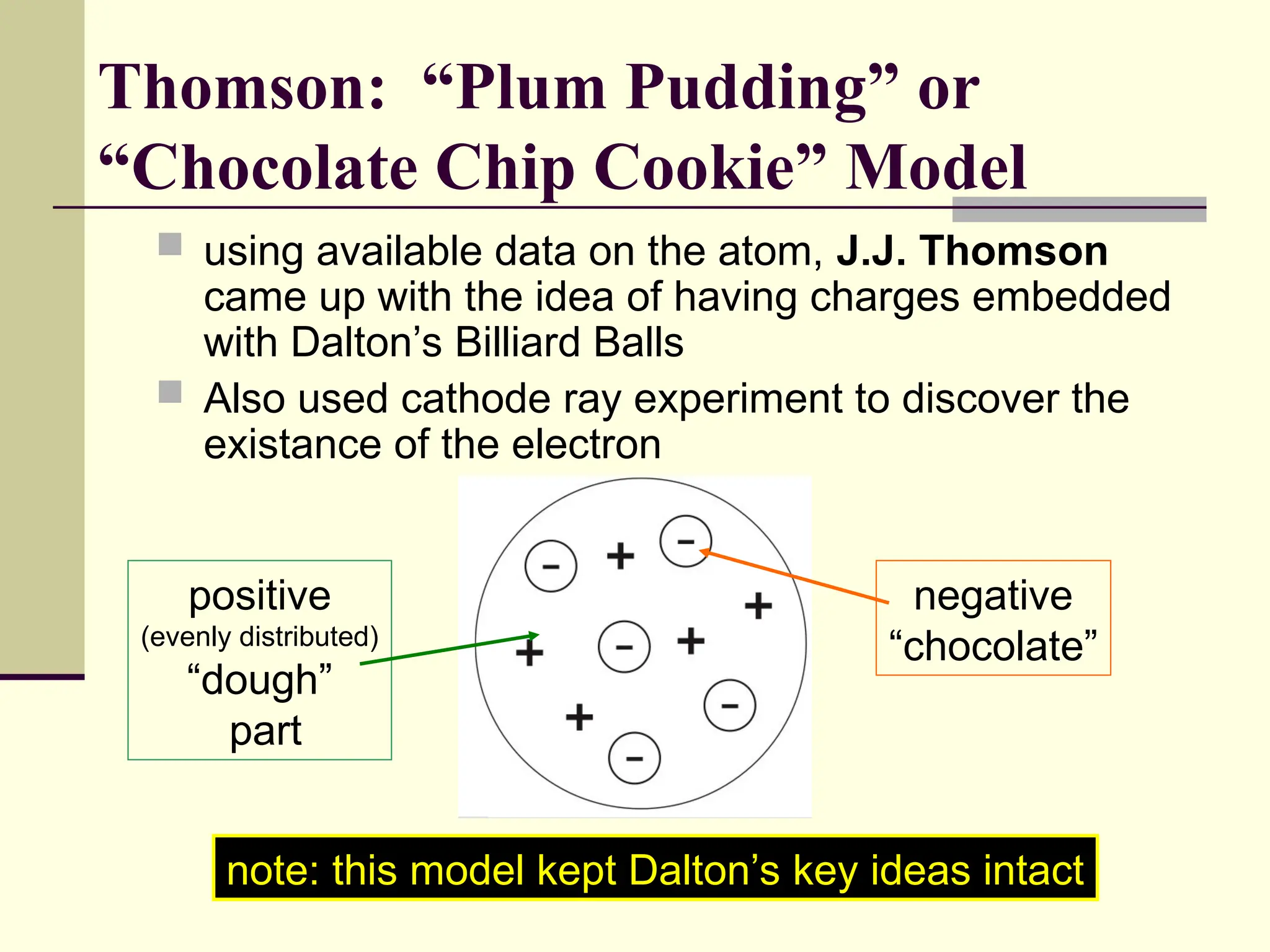
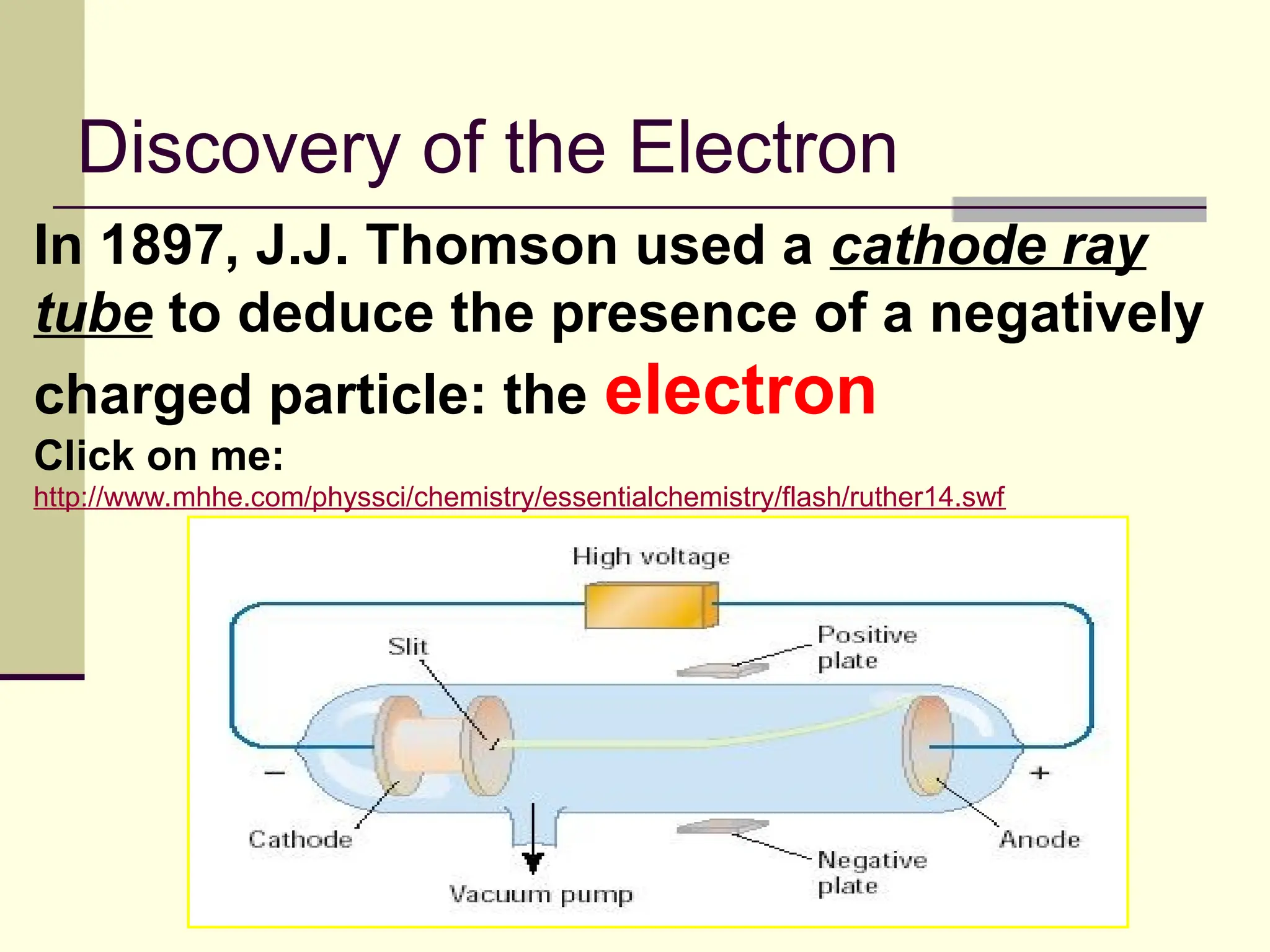
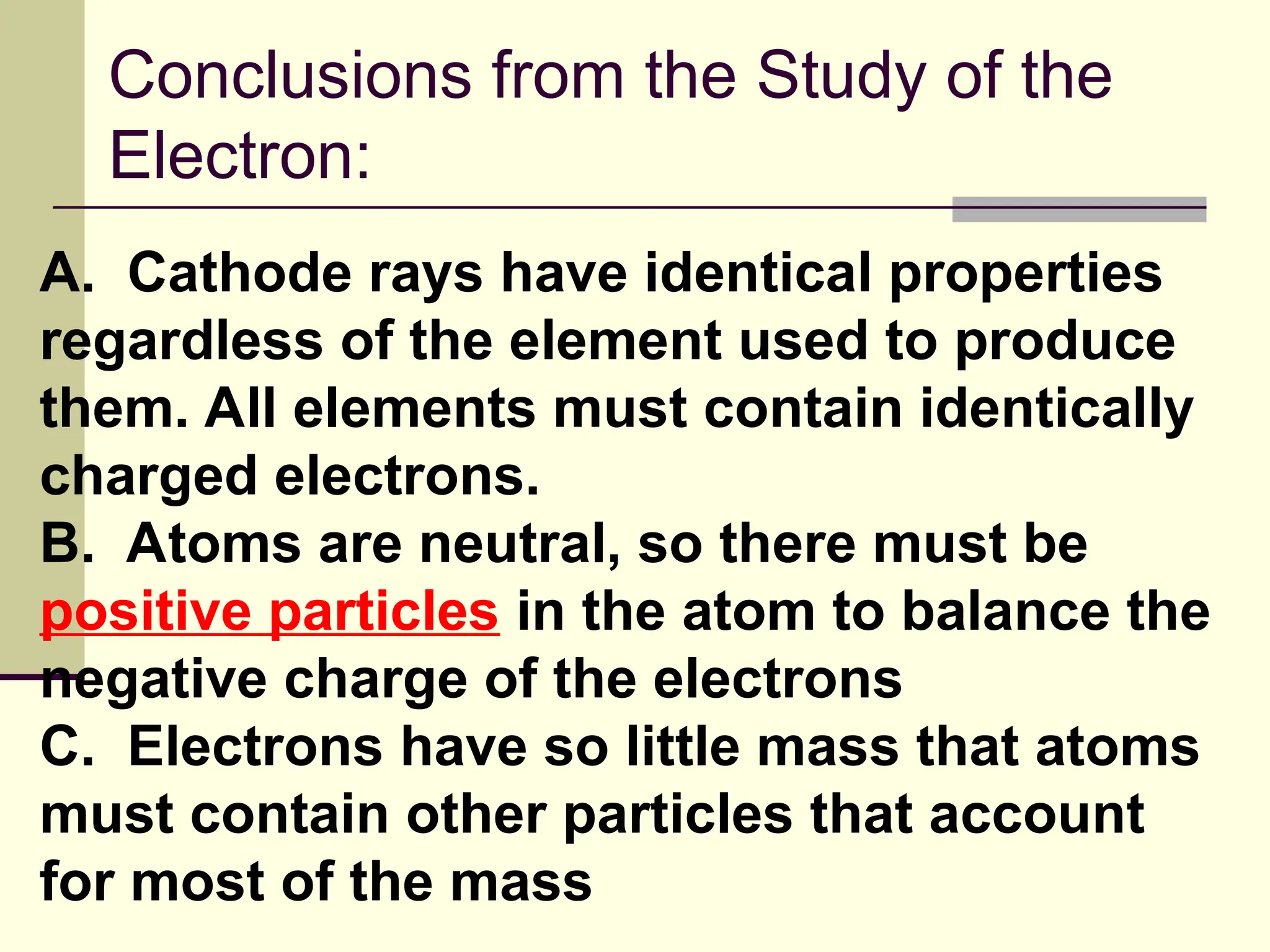
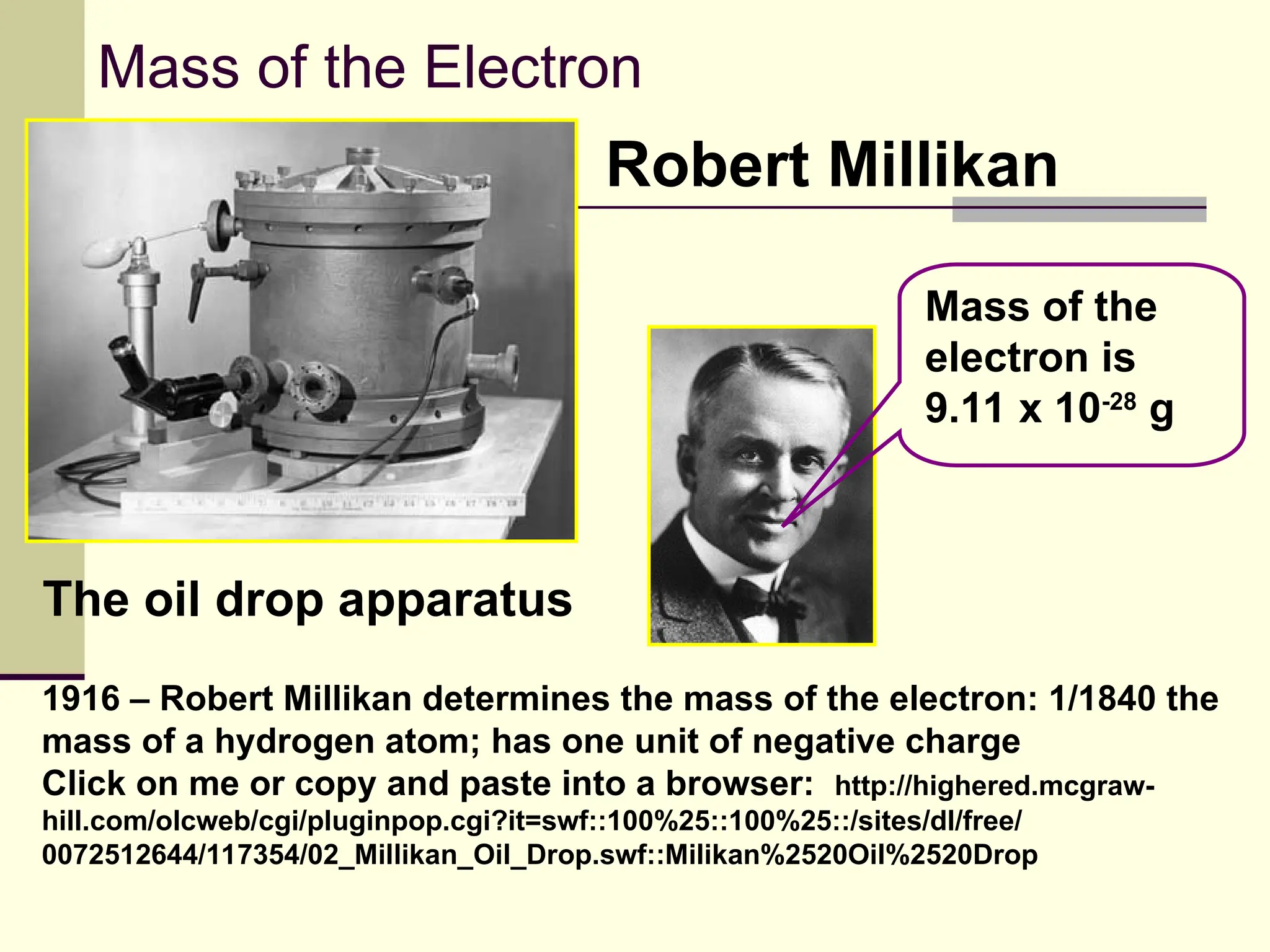
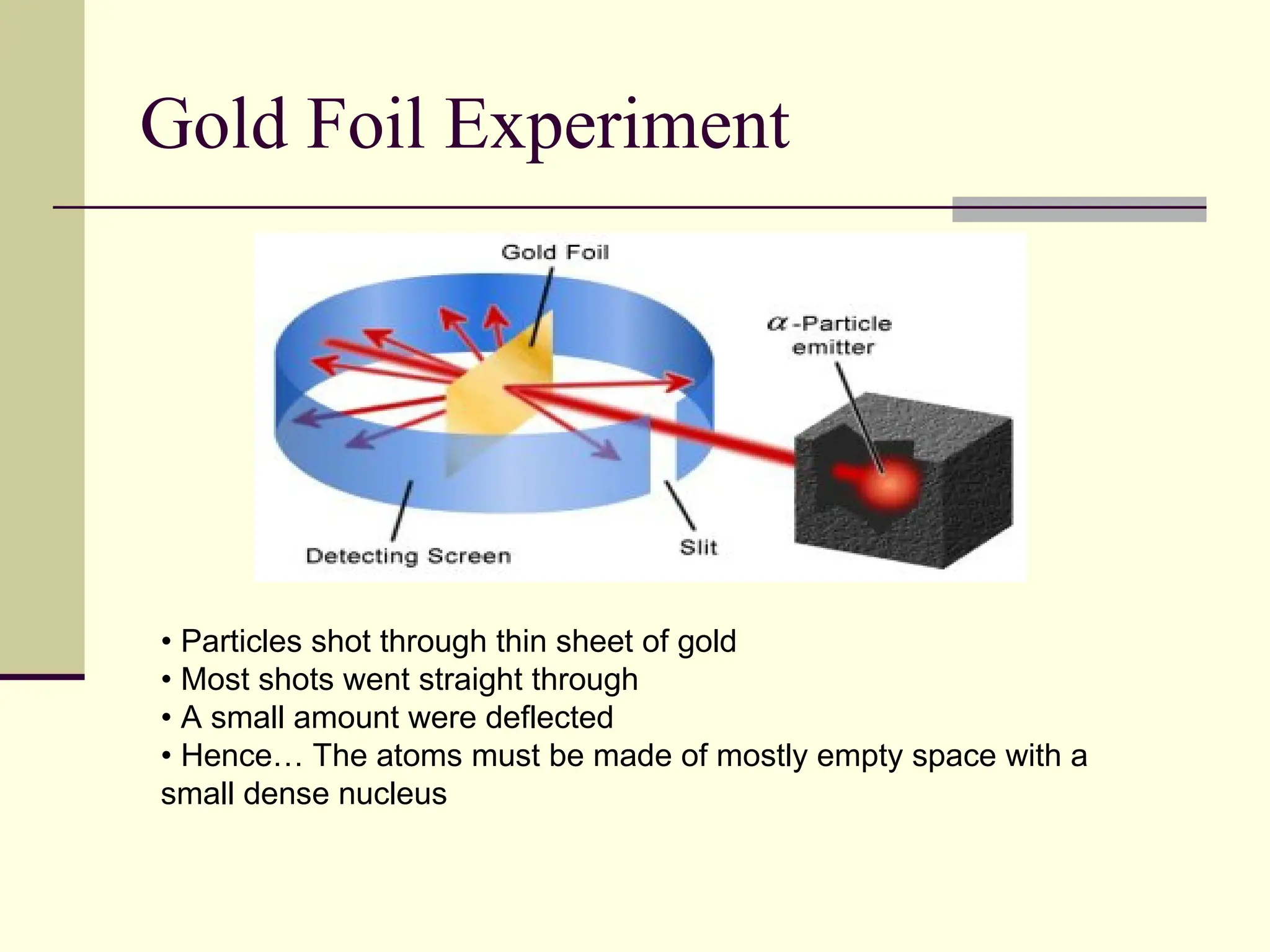
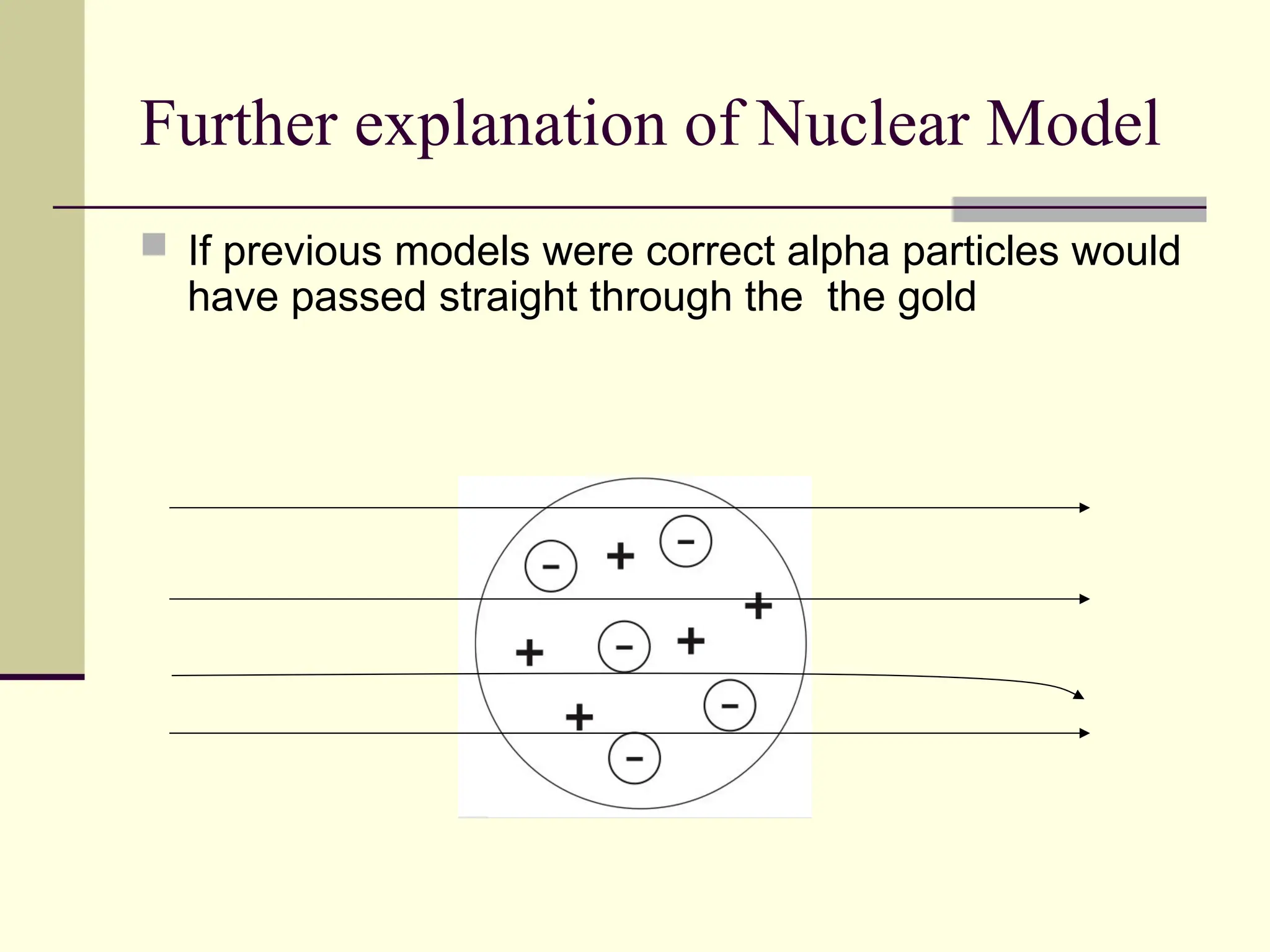
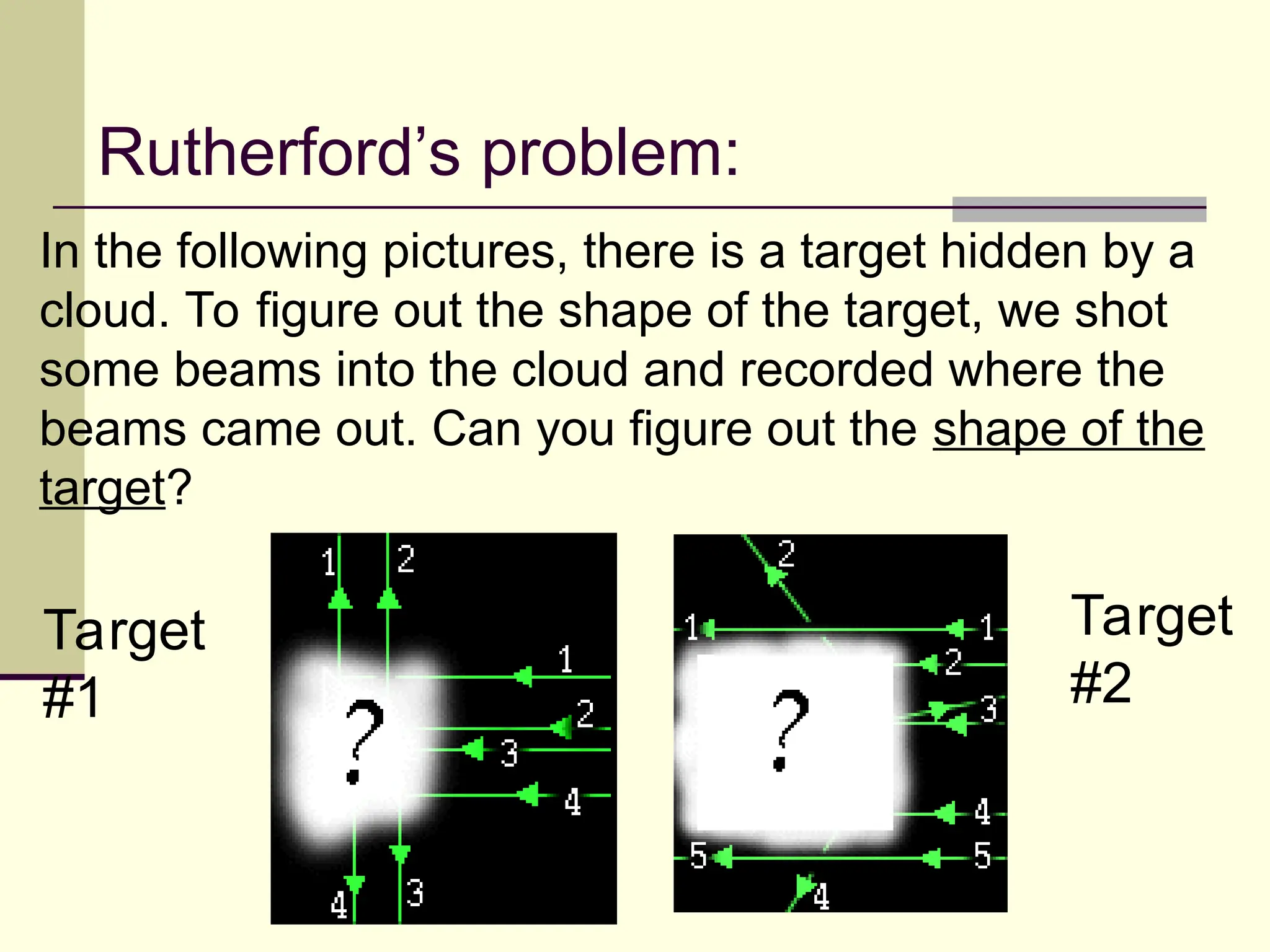
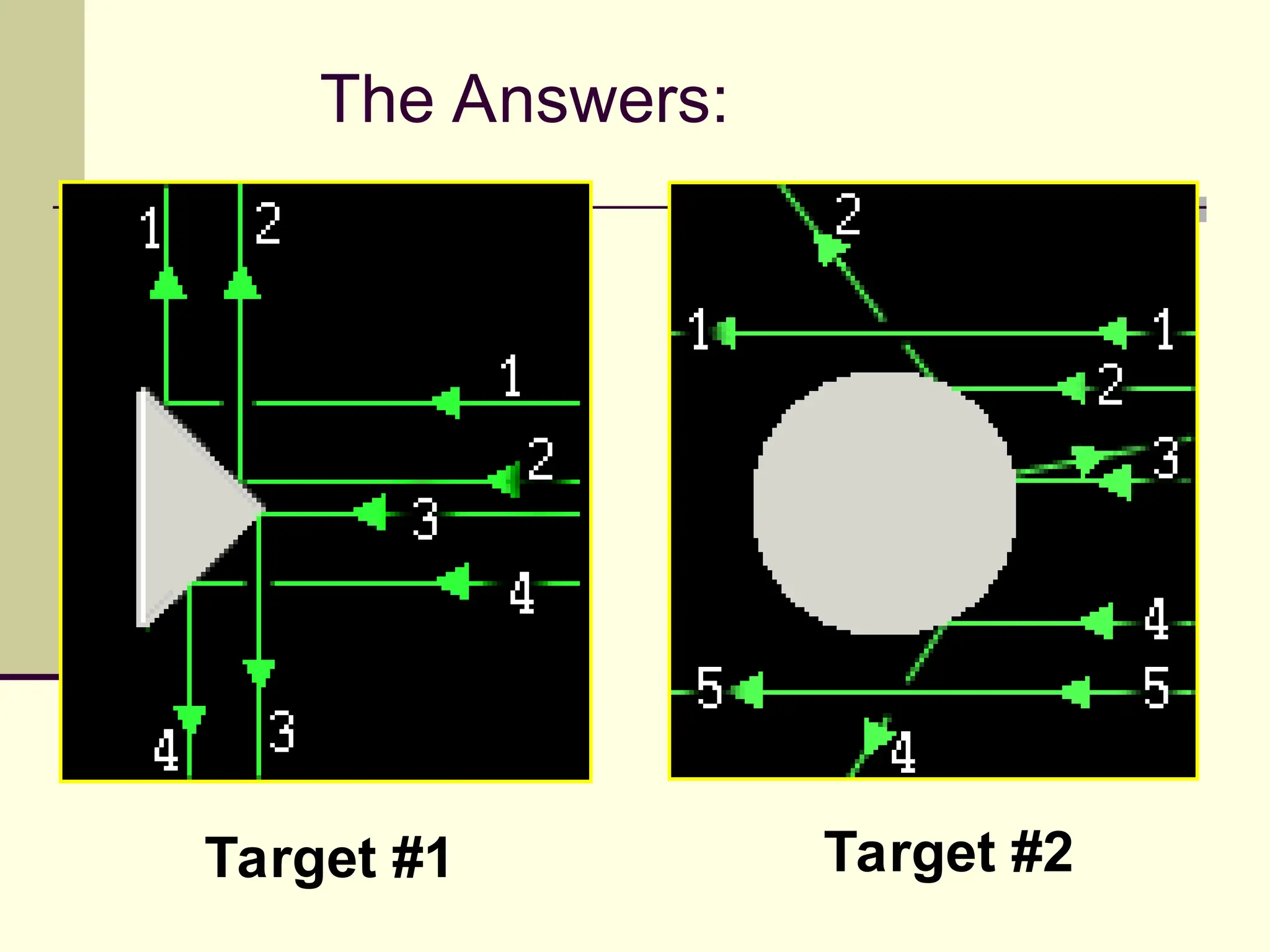
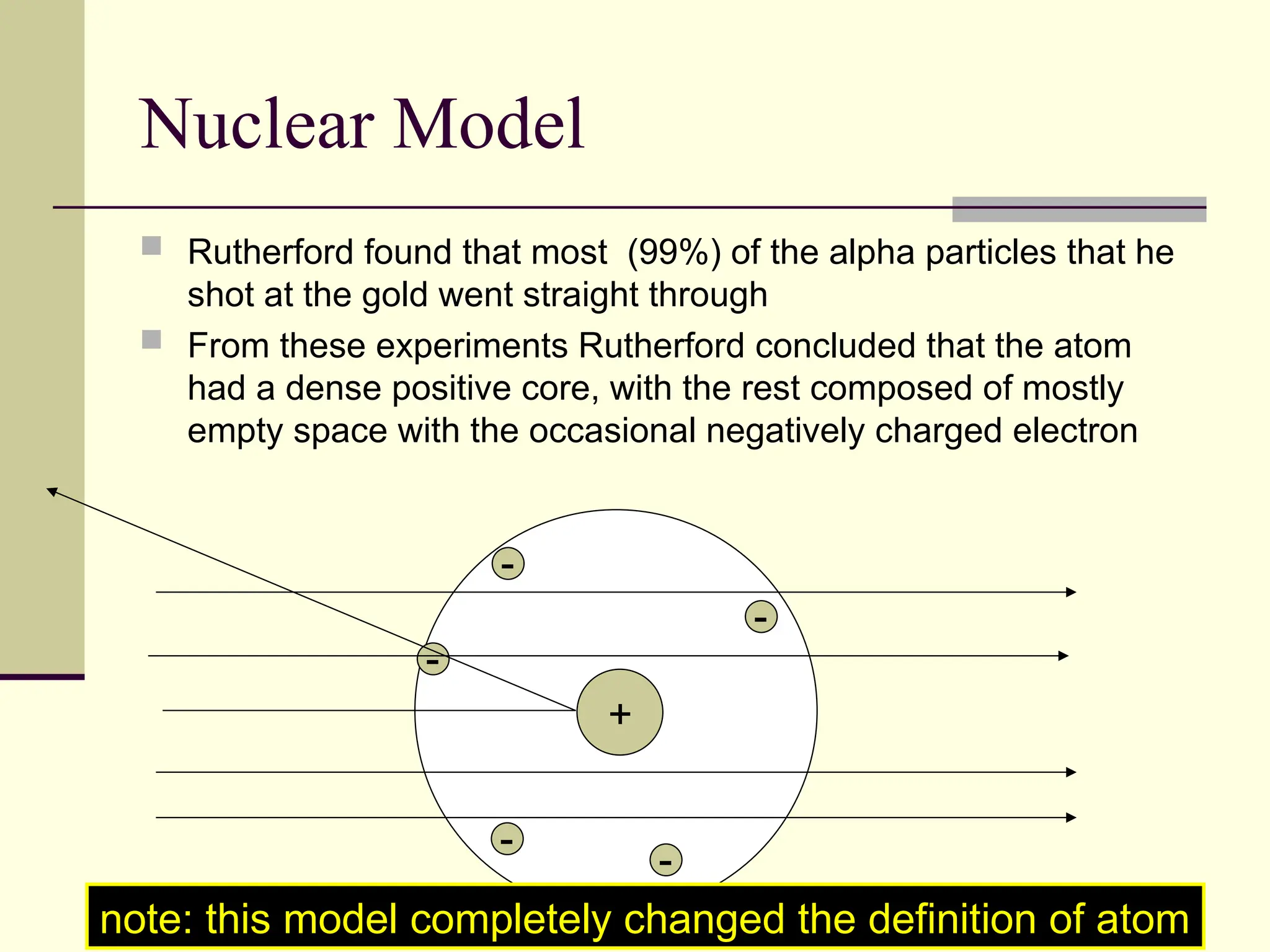
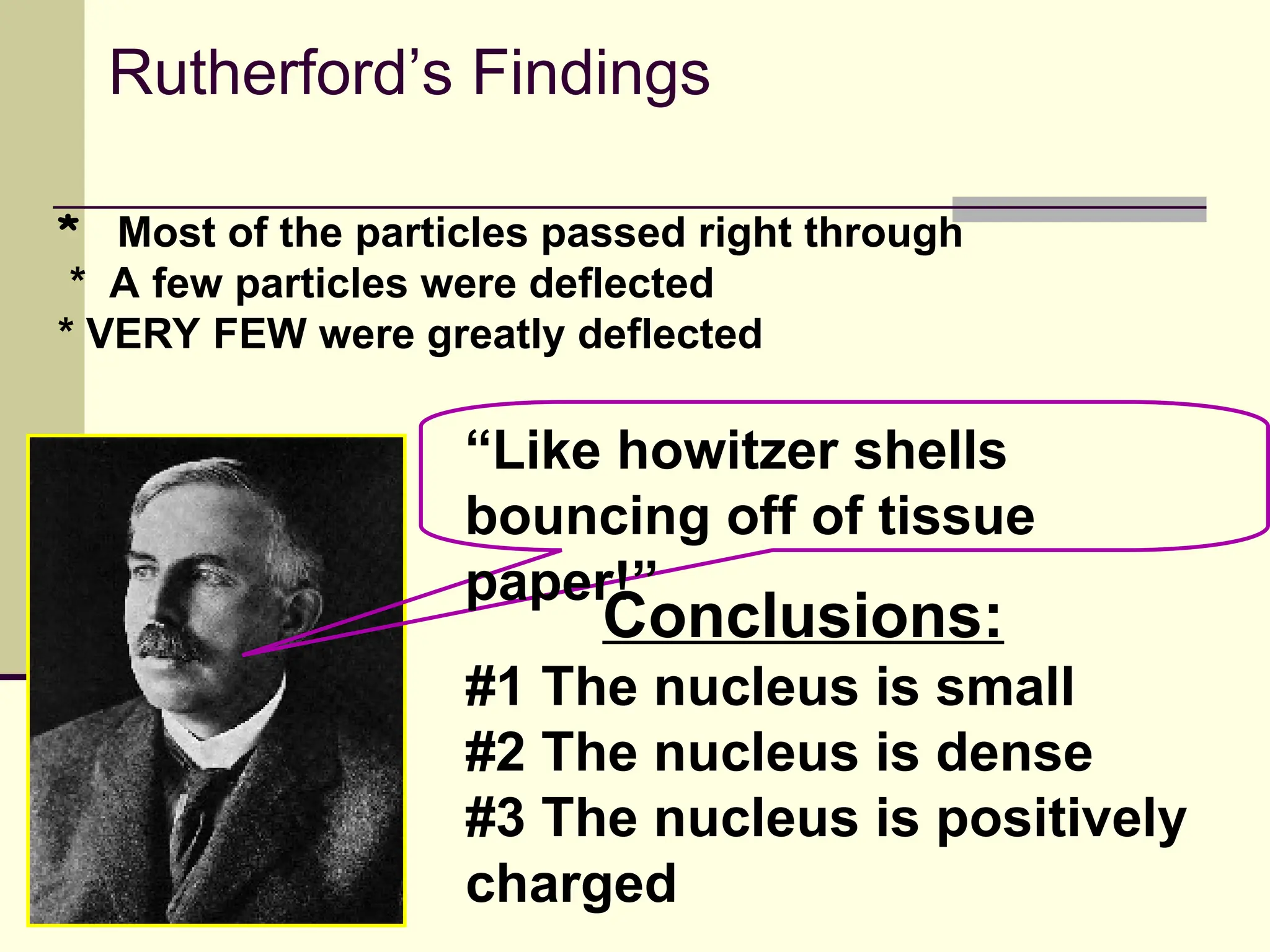
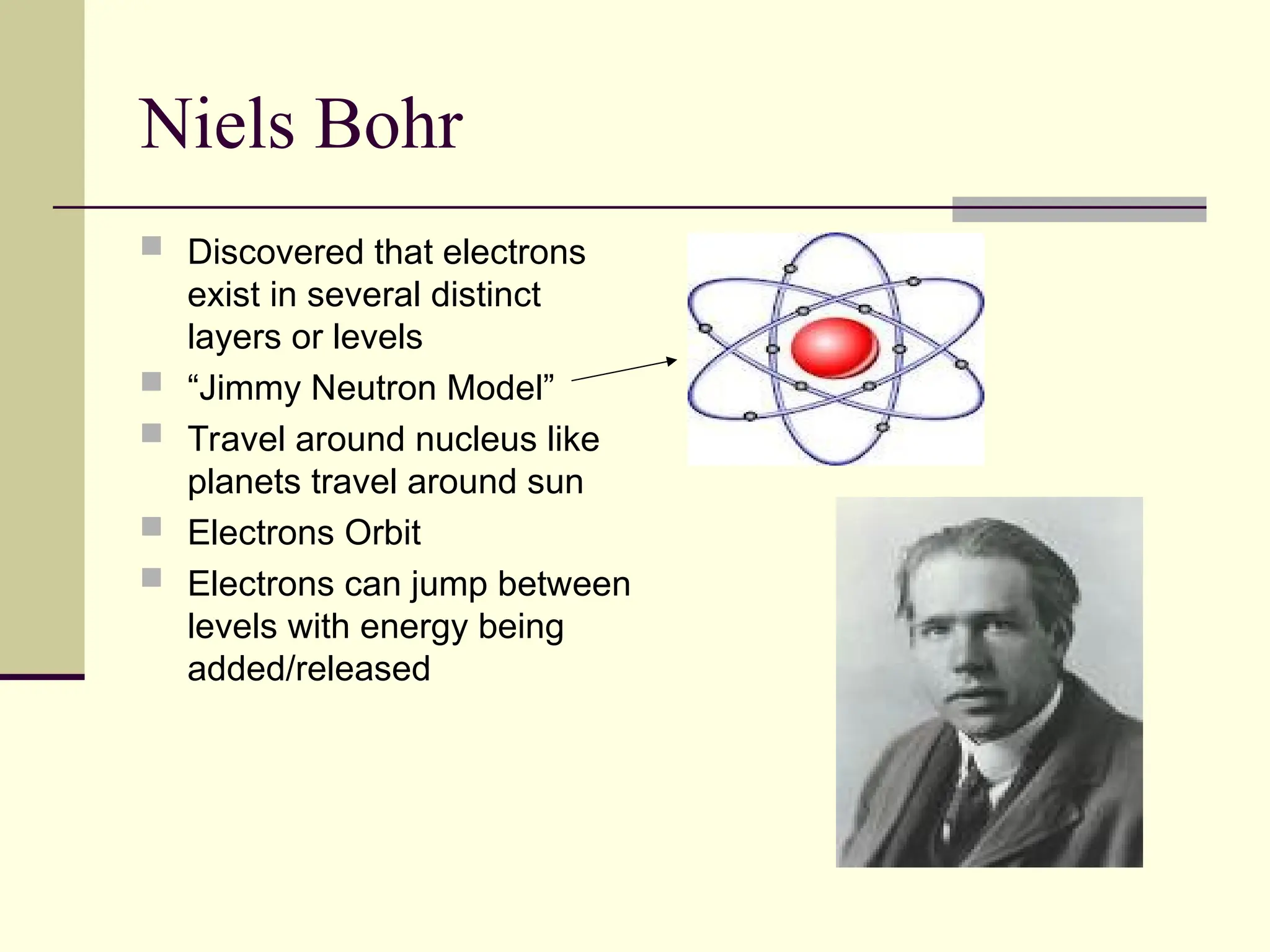
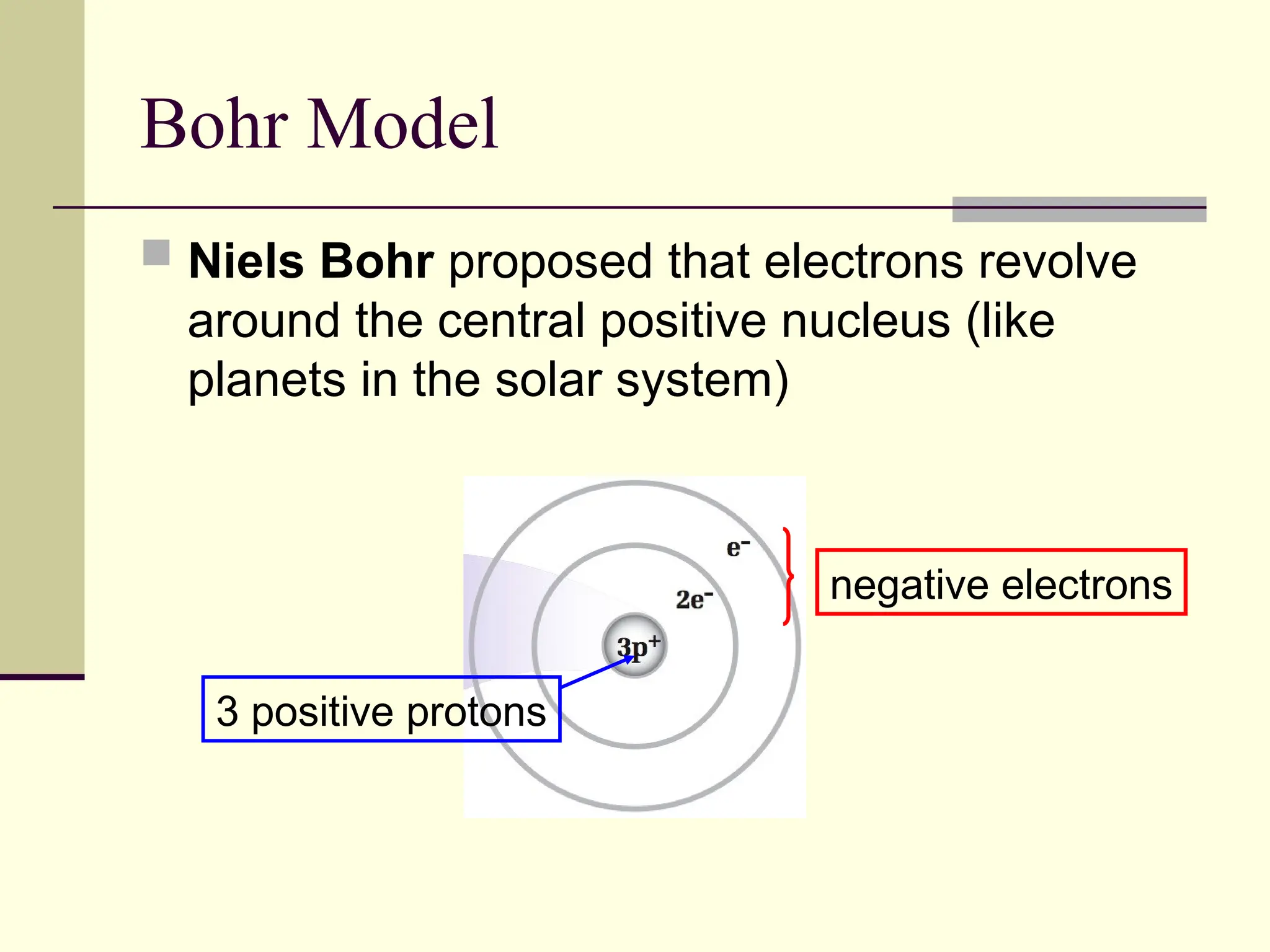
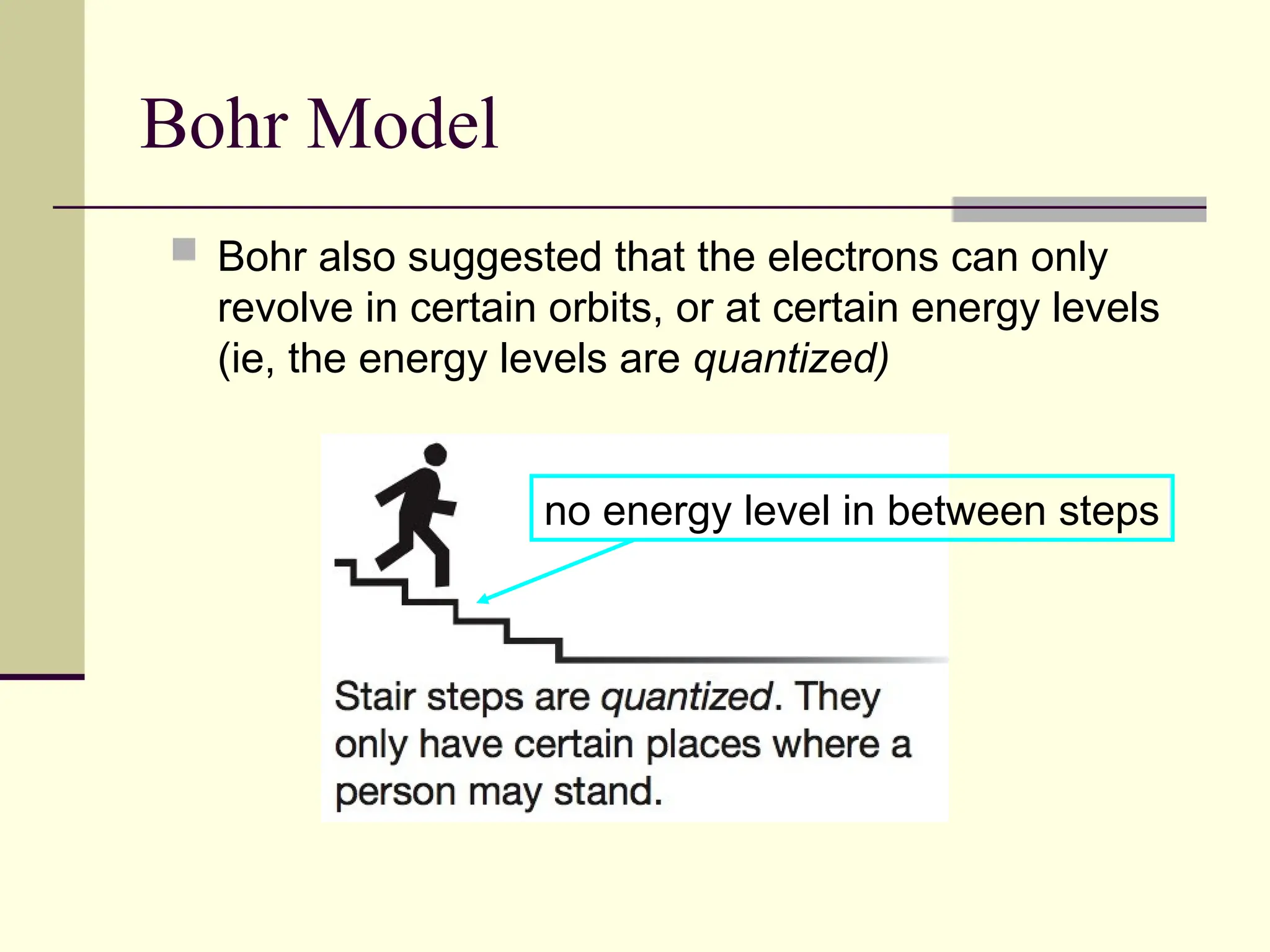
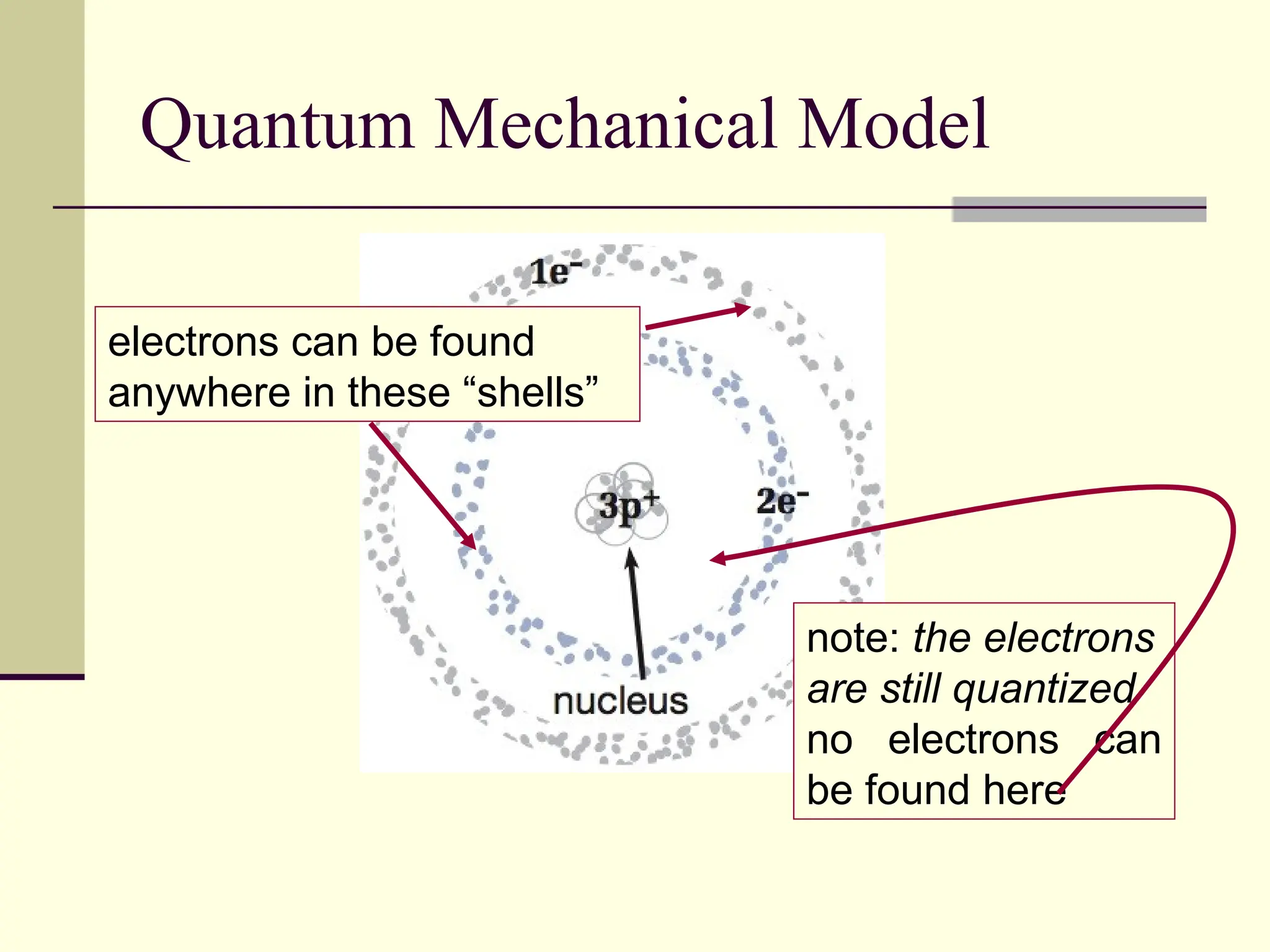
The document outlines the historical development of atomic theory, beginning with Democritus' idea of atoms and John Dalton's atomic theory, which evolved with contributions from J.J. Thomson's 'plum pudding' model and Ernest Rutherford's discovery of the nucleus. It discusses advances in understanding atomic structure, including the introduction of isotopes by Frederick Soddy and the quantum mechanical model developed by Heisenberg and Schrodinger. The document highlights key experiments and theories that shaped our current understanding of atomic structure and behavior.