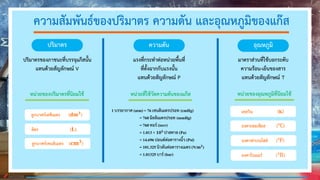
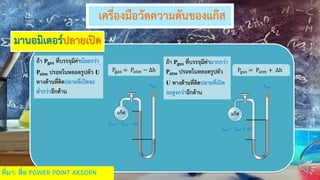
The document discusses properties of gases and their relationships based on gas laws. It explains that gas particles are far apart with little attraction between them, causing gases to spread evenly and take the shape and volume of their container. The volume, pressure, and temperature of gases are interrelated based on Boyle's law, Charles's law, Gay-Lussac's law, and the combined gas law.