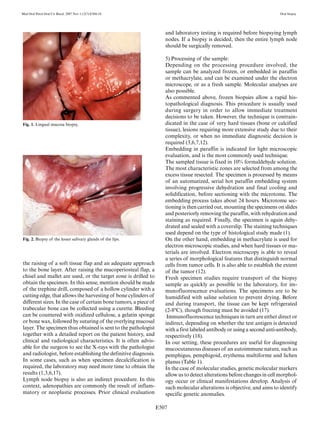
This document discusses oral biopsies in dental practice. It defines what an oral biopsy is and explains the different types of biopsies including incisional, excisional, and punch biopsies. It outlines the appropriate uses of biopsies for diagnosing oral lesions and diseases. Key indications for oral biopsies include persistent oral mucosal lesions, radiotransparent bone lesions suggestive of malignancy, cysts, and lesions of infectious or systemic diseases. Contraindications include very ill patients and lesions in deep or difficult to access areas. The document reviews biopsy techniques, materials, timing, and locations.