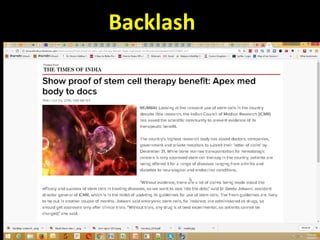
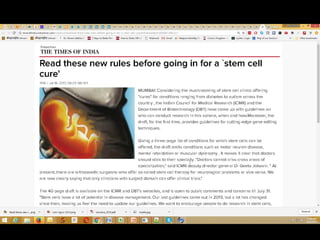
The document discusses national guidelines for stem cell research in India from 2013 and draft guidelines from 2017. The 2013 guidelines only approved stem cell transplantation for blood disorders and required all other uses to be in clinical trials. The 2017 draft guidelines recognize potential benefits of stem cells but stress the need for safety and efficacy data before clinical use. It warns against unregulated stem cell therapy due to lack of evidence, patient exploitation, and safety risks like tumor growth. The draft provides recommendations for quality control of cell processing and manufacturing.