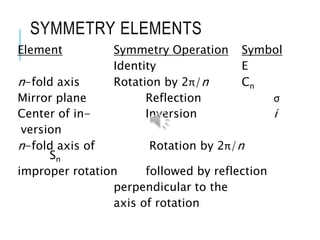
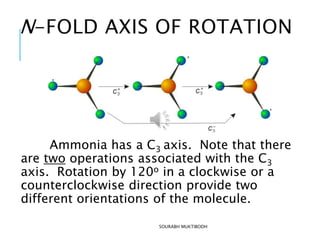
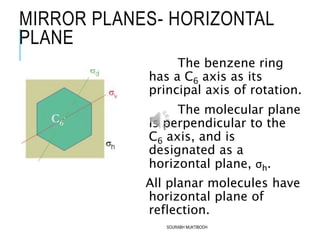
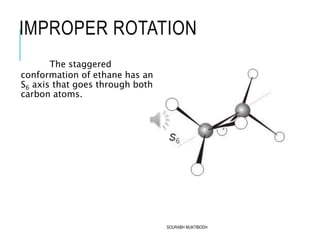
The document discusses symmetry elements and operations in molecular chemistry, highlighting their significance in predicting vibrational spectra, hybridization, and optical activity. It defines and categorizes different types of symmetry elements, including identity, proper rotation, mirror symmetry, and improper rotation, while providing examples and graphical representations. The summary concludes that understanding these symmetry elements allows for the identification of molecular point groups, facilitating the application of group theory in chemistry.