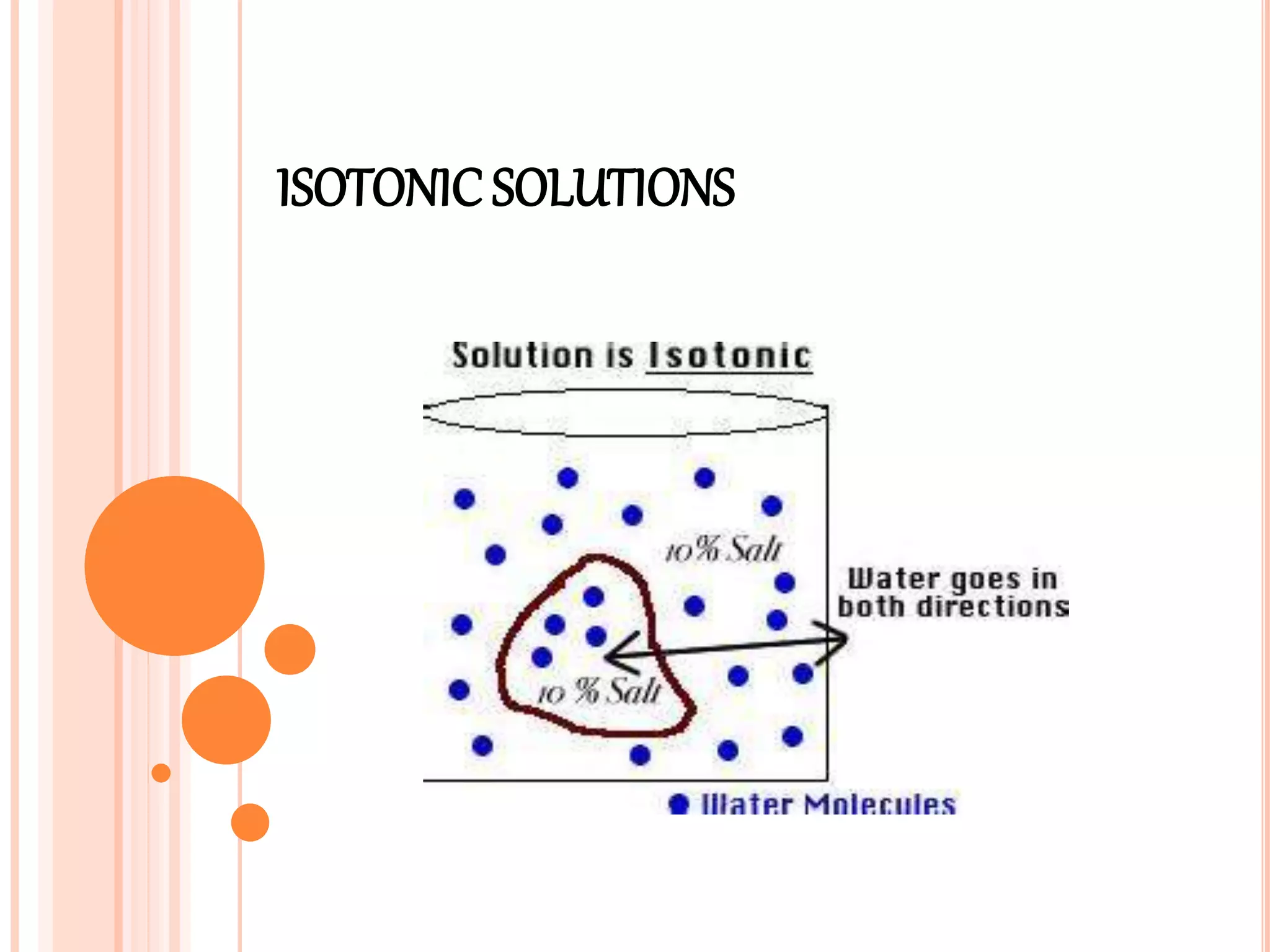
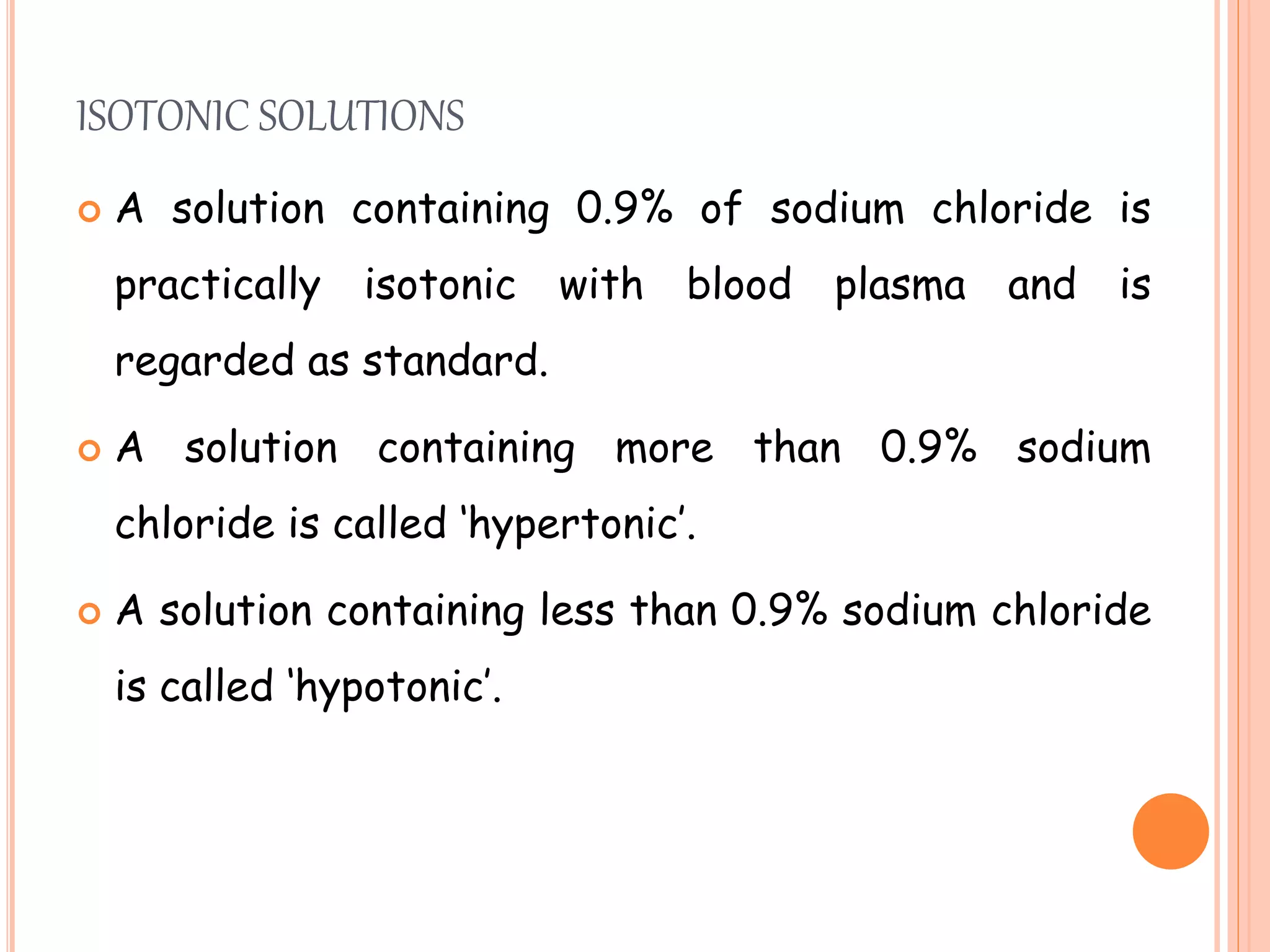
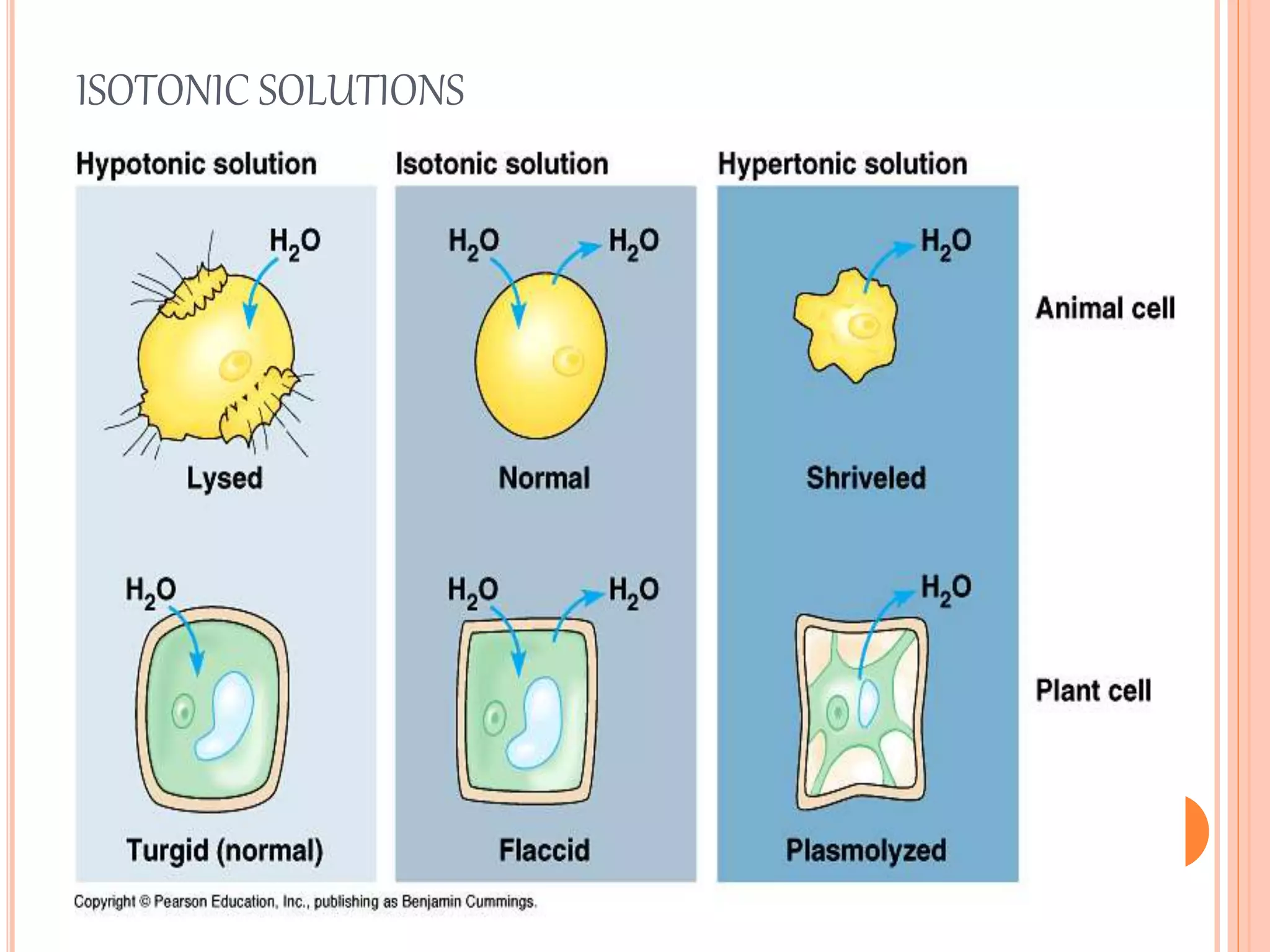
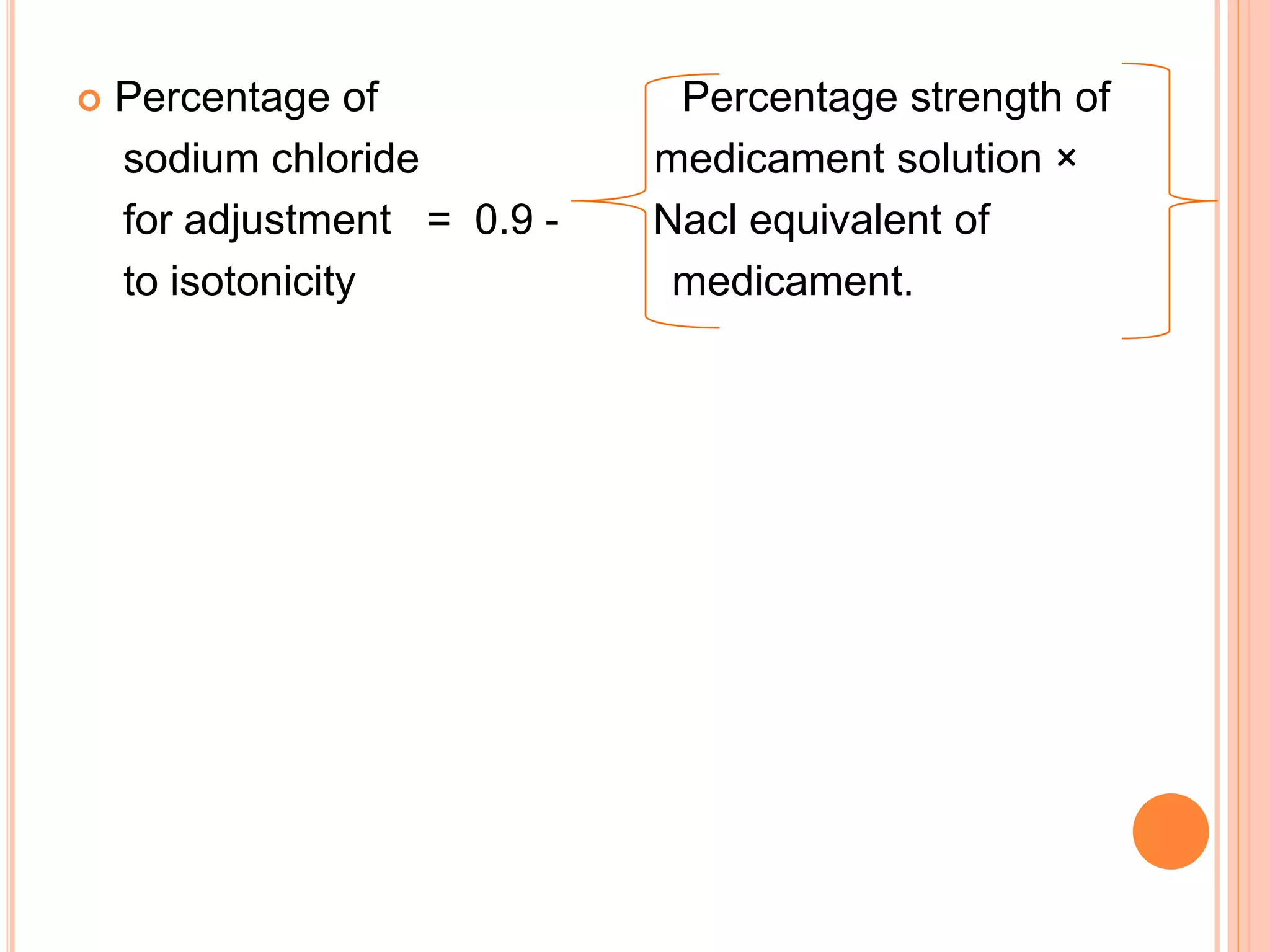
This document discusses isotonic solutions and how to calculate concentrations needed for isotonicity. A 0.9% sodium chloride solution is isotonic with blood plasma. Solutions with higher sodium chloride concentrations are hypertonic, and those with lower concentrations are hypotonic. The document outlines several methods to calculate concentrations of substances needed to achieve isotonicity based on freezing point depression, molecular concentration, vapor pressure graphs, and sodium chloride equivalents. Parenteral preparations should generally be isotonic, while other routes like subcutaneous may not require isotonicity.