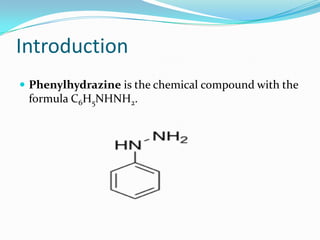
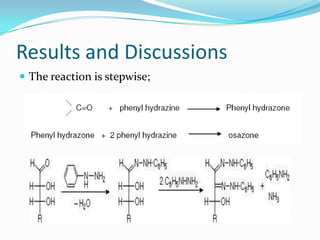
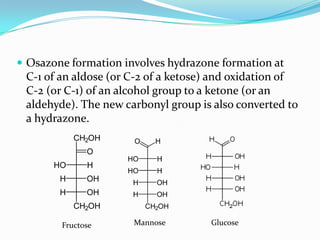
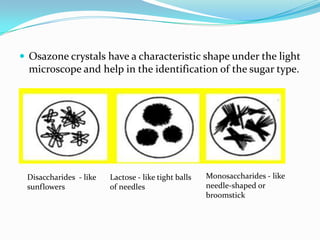
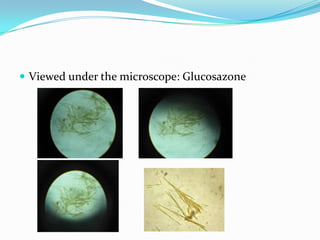
This document describes the osazone test, which is used to identify sugars. The test involves reacting sugars with phenylhydrazine at boiling point to form osazone crystals. Different sugars form characteristic crystal shapes that can be examined under a microscope to identify the type of sugar. The time it takes for crystals to form also helps in identification, with fructose forming crystals within two minutes while galactose takes 15-19 minutes. The test is useful for identifying reducing sugars.