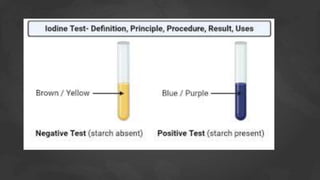
The document provides information about various carbohydrate tests that can be used to identify different types of carbohydrates. It describes the procedures for Molisch's test, Benedict's test, Nylander's test, Osazone crystal formation test, Tollen's test, Barfoed's test, Seliwanoff's test, and iodine test. Each test procedure involves adding a specific reagent to carbohydrate samples and observing any color changes or precipitate formations that indicate the presence of certain carbohydrates like glucose, fructose, maltose, or starch. The document also lists the general materials needed to perform the carbohydrate identification tests.