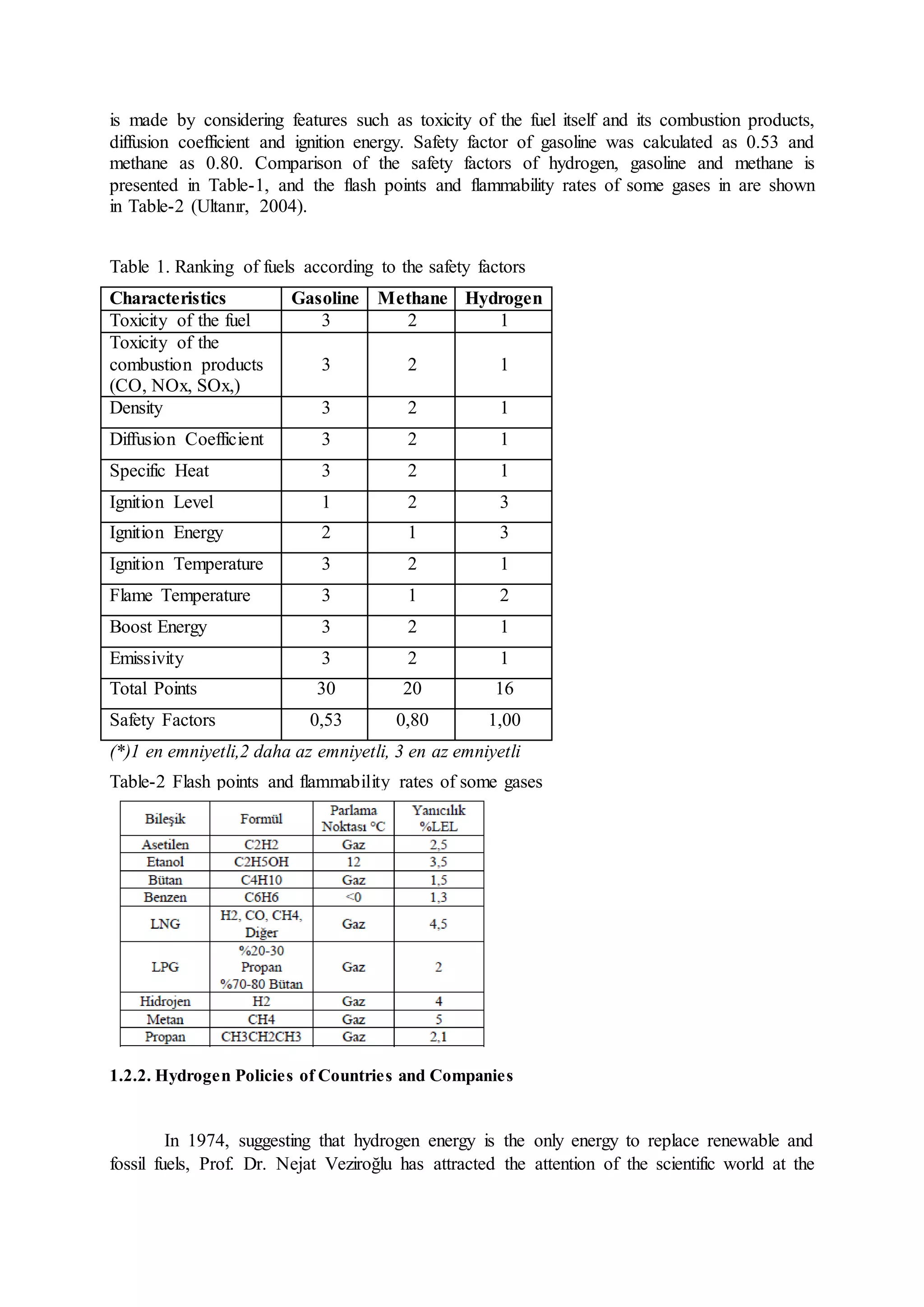
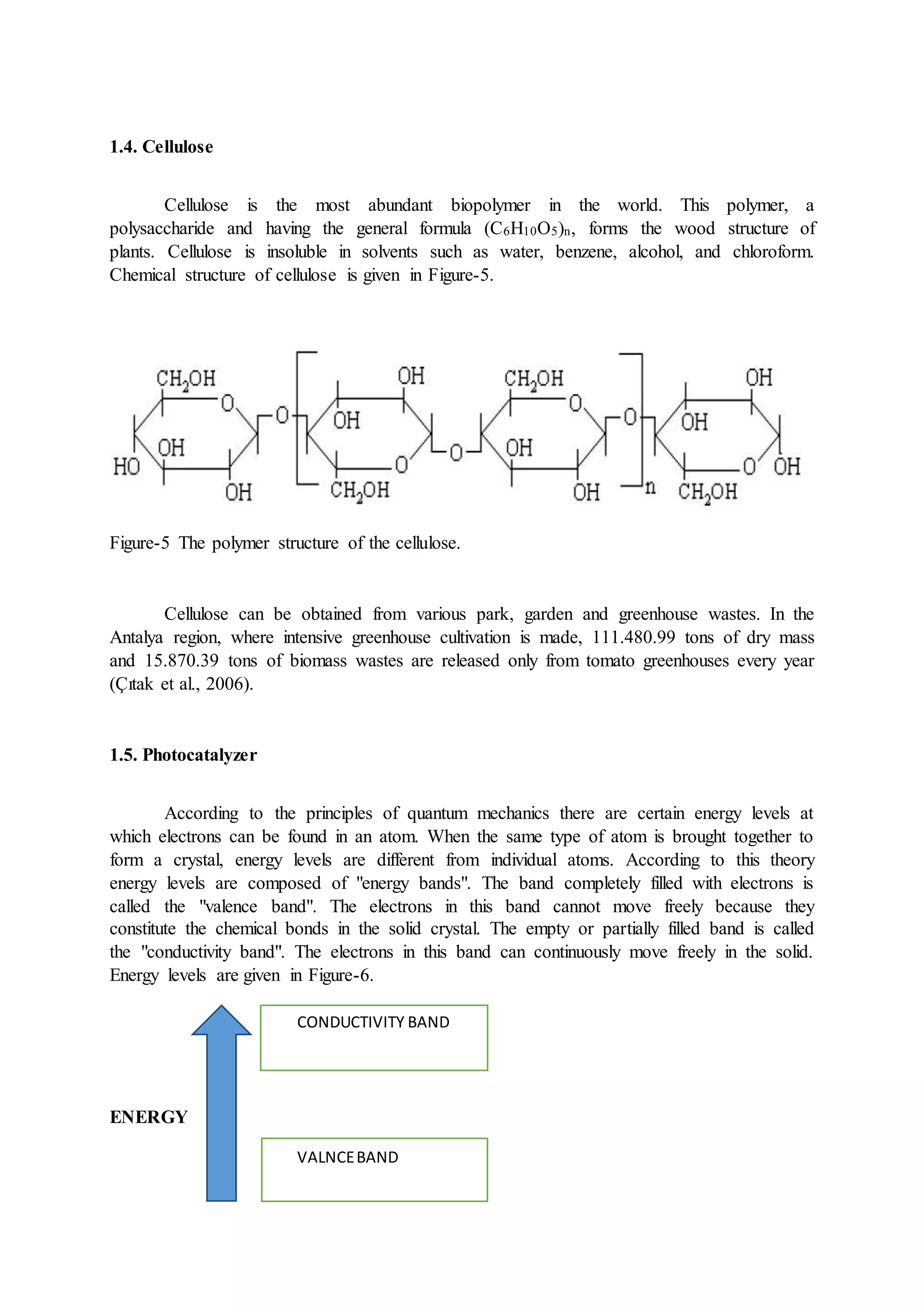
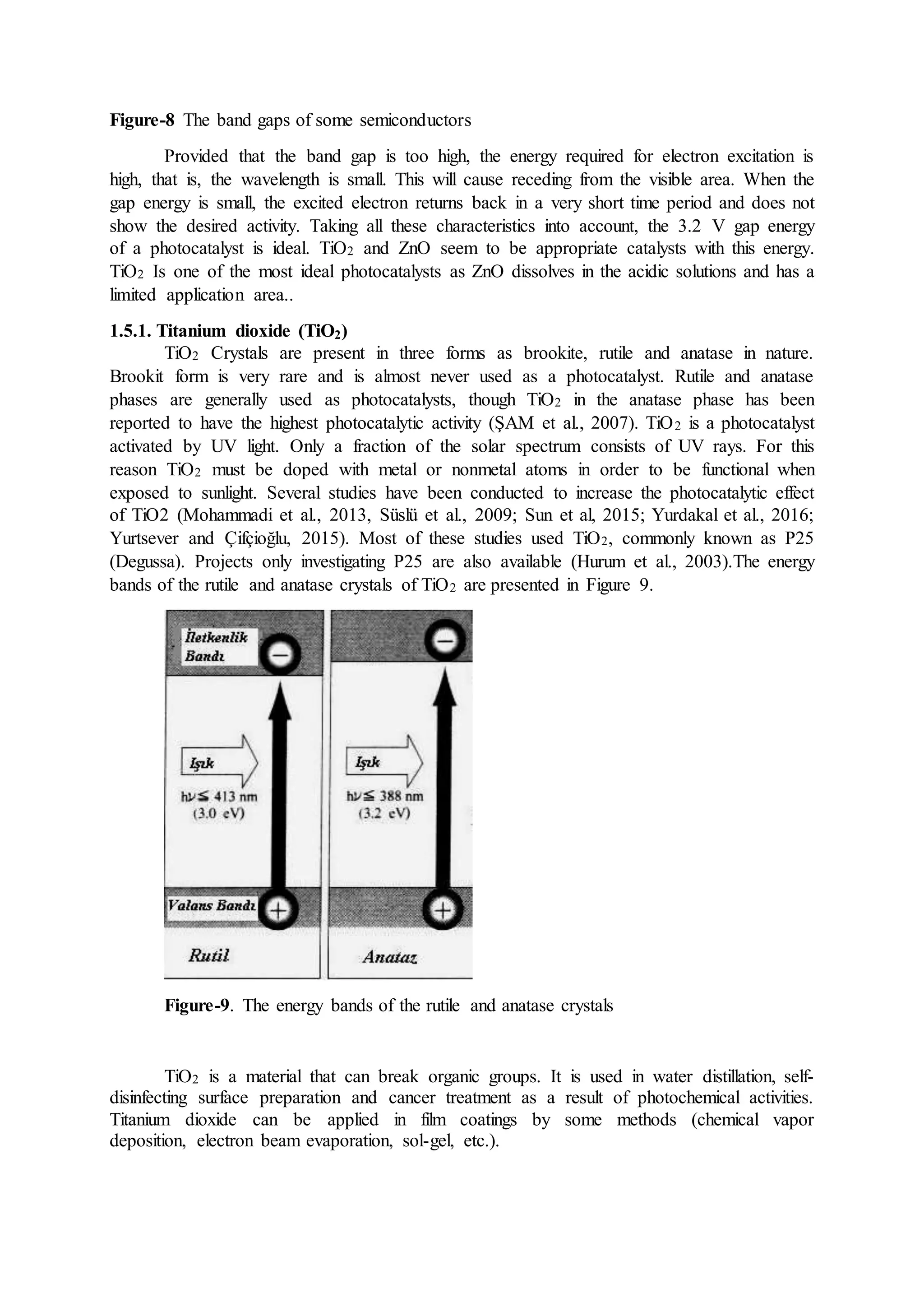
This document discusses hydrogen as a future energy source and outlines methods for its production. It first provides background on hydrogen as the most abundant element in the universe and discusses its properties that make it a promising fuel, such as having the highest energy content per unit mass of all fuels. The document then describes various methods for producing hydrogen, including from water via electrolysis or thermochemical processes, and biologically from biomass. It also outlines countries' and companies' policies supporting research and development of hydrogen fuel cell technologies.

















![tons of hydrogen gas can be produced annually from solely tomato and eggplant wastes
in Antalya with the help of solar energy. It is noteworthy to mention that the energy
equivalent is 4365 tons of fossil oil.
* Air pollution problem will also be solved when hydrogen is used as a fuel since H2O is
alone the the reaction product. Furthermore, the condensation of that water, produced
in the system, would also be a partial solution for another environmental problem: the
water supply problem. Thus, a big step will be taken FOR A BETTER LIFE.
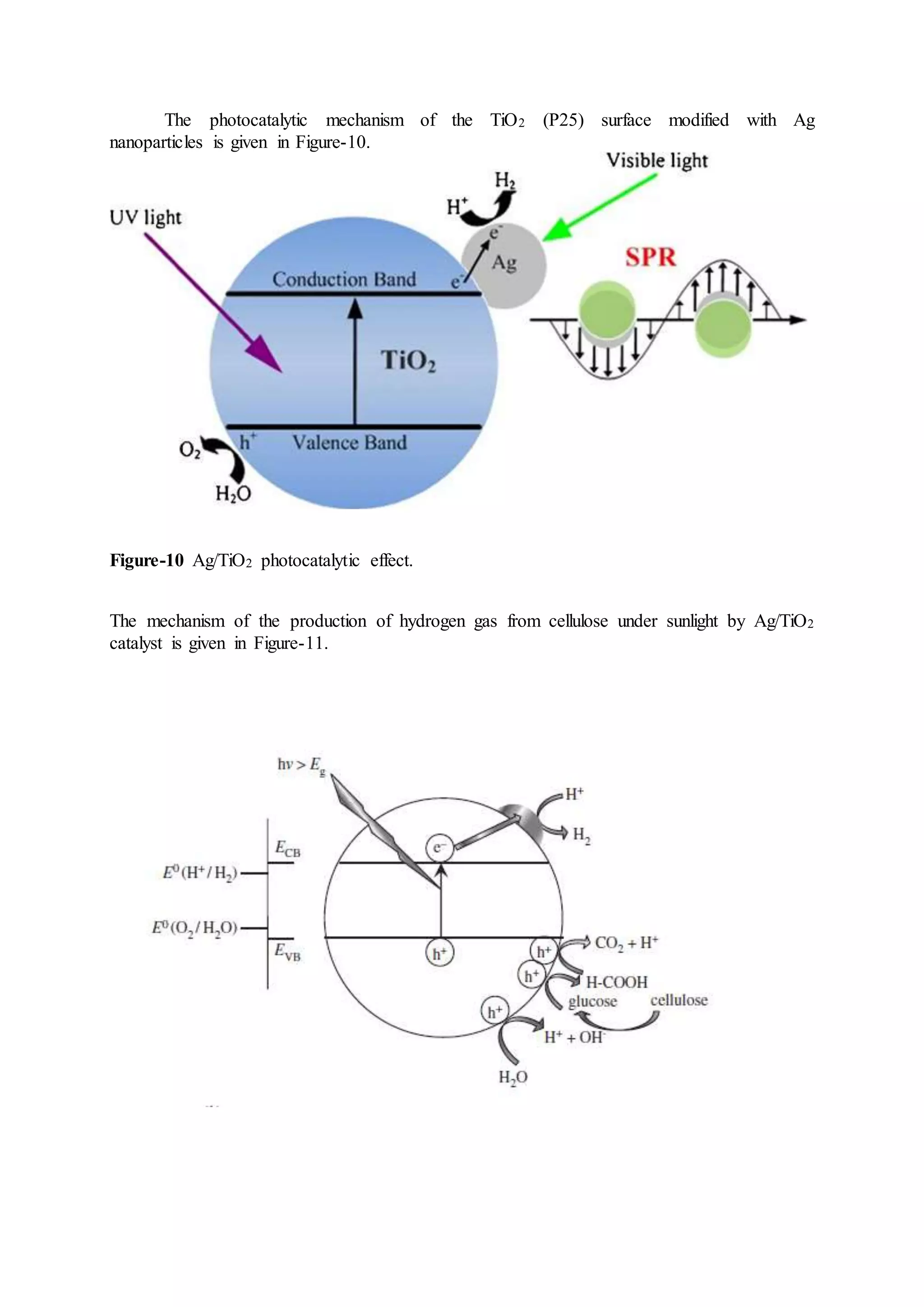
* In previous studies, TiO2 surface was doped with Ag; however, not used for the production
of hydrogen from cellulose. (Ornate, 2013; Yurdakal, 2016). Additionally, TiO2 crystal was
doped with Pt, Pd, Ni and Au, and the experiment was carried out at 60 °C to produce
hydrogen gas from the cellulose (Caravaca, 2016). In our project, on the other hand,
experiments were carried out under room conditions; that is, without additional heating. The
production of hydrogen gas from cellulose with modified Ag/TiO2 surface would be a
pioneering work in the literature.
5. COMMENTS
* The experiment was carried out at 60 °C in the previous studies. We performed the experiment
under room conditions. The effect of temperature can further be investigated by comparing the results
obtained at different temperatures.
* We used % 1 Ag/TiO2 photocatalyst. The effect of photocatalyst can be studied by changing the
Ag/TiO2 ratios.
6. REFERENCES
Anonim, 2016, İnternet haber, (son erişim tarihi: 02.01.2017)
http://www.dunyabulteni.net/haberler/367347/almanyanin-benzinli-otomobil-yasagi-
resmilesti
Amendola S.C., Sharp-Goldman S.L., Janjua M.S., Kelly M.T., Petillo P.J., Binder M., 1999 ,
"An Ultrasafe Hydrogen Generator: Aqueous, Alkaline Borohydride Solutions and Ru
Catalyst",Journal of Power Sources, 85,(2000)186-189
Aslan Ö., 2007, ‘Hidrojen Ekonomisine Doğru’İstanbul Ticaret Üniversitesi Sosyal Bilimler
Dergisi 6(11) ,283-298
Atkinson B., Roth S., Hirscher M., Griinwald W., 2001,’’Carbon nanostructures; An efl]cient
hydrogen storage medium for fuel cells?’Fuel Cells Bulletin,Germany No:38, 9-12
Benli Y., 2014,‘Gümüş İle Doplamış Nano-TiO2’in Özelliklerinin İncelenmesi’(Doktora tezi)](https://image.slidesharecdn.com/hydrogenreport-170515200607/75/SciChallenge2017-Hydrogen-report-22-2048.jpg)

