Lecture 2 MICROSCOPY.pptx
170male reproductive systemmale reproductive system xtestis is covered by three layers (from outside to inwards): ̞visceral layer of tunica vaginalis: ̎it is lined by flat mesothelial cells.ons of seminiferous tubules lined by spermatogonia, primary and secondary xThese tight junctions form the blood–testis barrier. xThe tight junction divides the intercellular compartment between the Sertoli cells into basal and luminal compartment. xBasal compartment contains spermato gonia and primary spermatocytes. xLuminal compartment contains secondary spermatocytes and spermatids (Fig. 19.5). Functions of Sertoli Cells xSertoli cells provide support and nutrition to spermatogenic cells. xThe bloodtestis barrier protects the spermatogenic cells from the harmful substances (antigens) of blood. xThey phagocytose the residual bodies. xSertoli cells secrete androgen-binding protein (ABP), which concentrates the testosterone. xIn fetal testis, Sertoli cells produce anti mullerian hormone, which inhibits the development of mullerian duct. xSertoli cells are nondividing cells, highly resistant to infection, malnutrition, and radiation. xThese produce inhibin, which inhibits the secretion of follicle-stimulating hormone (FSH). Interstitial Cells of Leydig xThese are large polyhedral cells lying in the connective tissue between seminif erous tubules. xThese are pale staining cells with eccen tric nucleus and cytoplasm shows unique needleshaped crystalline inclusion (Reinke’s crystal). spermatocytes, spermatids, and sperms are seen. 2.Sertoli cells are seen in between the spermatogenic cells. 3.Interstitial ces of Leydig are seen in between the seminiferous tubules. xThey secrete testoster ̞tunica albuginea: ̎it is a thin layer of connective tissue containing collagen, blood vessels, and lymphatics. ̎along the posterior border, tunica albuginea is thickened to form medi astinum testis. ̎septa arising from the mediastinum testis divide the substance of the testis into 200 to 300 lobules. ̎each lobule contains one to four seminiferous tubules. ̎seminiferous tubules contain coiled part in the front and straight part behind. ̎straight part enters the medi astinum testis where it joins and forms a network called as rete testis. ̎from the upper end of rete testis 12 to 14 efferent ductules arise and enter the epididymis. ̞tunica vasculosa: ̎highly vascularized connective tissue which covers the individual lobule. microscopic structure oftestis seminiferous tubule xthere are 400 to 600 seminiferous tubules in each testis. xeach tubule is surrounded by a basal lamina supported by connective tissue which contains muscle-like myoid cells. xcontraction of myoid cells helps to move the spermatozoa along the tubule. xeach seminiferous tubule is lined by stratified seminiferous epithelium which contains spermatogenic cells and sertoli cells(figs. 19.2and19.3). fig. 19.2diagram of testis (h&e pencil). h&e, hematoxylin and eosin. 3.Interstitia
Recommended
Recommended
More Related Content
Similar to Lecture 2 MICROSCOPY.pptx
Similar to Lecture 2 MICROSCOPY.pptx (20)
More from elphaswalela
More from elphaswalela (20)
Recently uploaded
Recently uploaded (20)
Lecture 2 MICROSCOPY.pptx
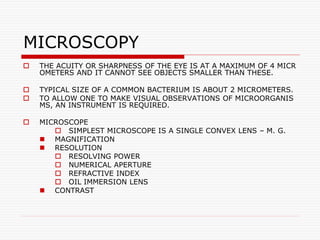
- 1. MICROSCOPY THE ACUITY OR SHARPNESS OF THE EYE IS AT A MAXIMUM OF 4 MICR OMETERS AND IT CANNOT SEE OBJECTS SMALLER THAN THESE. TYPICAL SIZE OF A COMMON BACTERIUM IS ABOUT 2 MICROMETERS. TO ALLOW ONE TO MAKE VISUAL OBSERVATIONS OF MICROORGANIS MS, AN INSTRUMENT IS REQUIRED. MICROSCOPE SIMPLEST MICROSCOPE IS A SINGLE CONVEX LENS – M. G. MAGNIFICATION RESOLUTION RESOLVING POWER NUMERICAL APERTURE REFRACTIVE INDEX OIL IMMERSION LENS CONTRAST
- 2. Types of microscopes Light Microscopy The light microscope uses visible light to detect s mall objects The following challenges are often encountered when using a light microscope obtaining sufficient contrast finding the focal plane obtaining good resolution recognizing the specimen when one sees it
- 3. Bright Field Microscopy Light from an incandescent source is aimed toward a lens b eneath the stage called the condenser This passes through the specimen, through an objective len s, and to the eye through a second magnifying lens, the ocu lar or eyepiece Objects are seen in the light path because natural pigmenta tion or stains absorb light differentially, or because they are thick enough to absorb a significant amount of light despite being colorless
- 4. A Paramecium should show up fairly well in a bright field micros cope However, it will not be easy to see cilia or most organelles The condenser is used to focus light on the specimen th rough an opening in the stage After passing through the specimen, the light is display ed to the eye with an apparent field that is much larger than the area illuminated The magnification of the image is simply the objective l ens magnification times the ocular magnification
- 5. Some condensers are fixed in position, others are focusable, so that the quality of light can be adjusted Usually the best position for a focusable condenser is as close t o the stage as possible The bright field condenser usually contains an aperture diaphra gm This is a device that controls the diameter of the light beam co ming up through the condenser When the diaphragm is stopped down (nearly closed) the light comes straight up through the center of the condenser lens an d contrast is high
- 6. When the diaphragm is wide open the im age is brighter and contrast is low A disadvantage of having to rely solely o n an aperture diaphragm for contrast is t hat beyond an optimum point the more c ontrast you produce the more you distort the image With a small, unstained, unpigmented sp
- 7. Brightfield Microscope Study size, shape & arrangement of microbia l cells, little information about internal cell structure. Tips: Molds & large protozoa-10x, 40x Bacteria,yeasts,small protozoa-100x oil immersion objective Increase amount of light when using th e 100x oil immersion objective lens
- 8. BRIGHTFIELD MICROSCOPE Allows light rays to pass directl y through the eye without bein g deflected by intervening opa que plate in the condenser.
- 10. Dark Field Viewing Dark-field Microscope (=Ultra-microscope) It was invented by Zsigmondy (1905) A special condenser lens is used with an opaque disc at the centre, so that direct rays don’t enter the objective lens Only light scattered by the specimen enter the objective len s to form a bright image against dark background Dark field microscope does not have a good resolution It is commonly used in microbiology
- 12. Darkfield Microscope Designed to eliminate the need for staining to achieve contrast between the specimen and the background. Condenser lens- focuses light o n the specimen at an oblique a ngle. Microorganisms appear very br ight on a dark background
- 14. Fluorescence Microscopy Allows the detection of molecules and ions within cells Fluorescent dyes absorb short wavelengths of light and emit longe r wavelengths Barrier filters and a dichroic prism select the excitation wavelengt h that strikes the specimen and exclude the excitation wavelength from the detector This allows only emitted light to reach the detector (oculars) uses uv light source = mercury or xenon arc lamp. - high contrast, high resolution image
- 15. special fluorescent dyes used to locate “mole cules” in a specimen - black background, bright-stained specimen - no condenser required, light comes from ab ove (“epi”) specimen - multiple fluorescent probes available - detects small quantities, molecules; can use antibody staining techniques
- 16. Fluorescence microscope Specimen is illuminated at one wa velength of light and observed by a light emittted at a different wavele ngth Fluorexcein isothiocyanate Excitation wavelength and emissio n wavelength Excitation filter and barrier filter
- 20. Fixed (Preserved) Specimens for Hist ology--the Study of Tissue 1) Specimens may be preserved using chemicals suc h as formalin, acetic acid, ethanol, and methanol Fixation immobilizes molecules such as proteins and lipids 2) Fixed specimens are dehydrated by serial transfer through an ascending alcohol series, to 100% alcoho l 3) Specimens are infiltrated with melted paraffin, pa raffin substitute, or plastic and placed in a mold to h arden
- 21. 4) Specimens are cut into 5-10 um thick sect ions using a steel knife or razor on an instru ment called a microtome 5) Sections are then mounted on slides, 6) Stained to achieve contrast or identify cell structures or components, and 7) Viewed microscopically
- 23. Many variations in technique are used to pre pare specimens for light microscopy Some omit the dehydration, infiltration, emb edding and sectioning steps and use aqueous staining systems for viewing whole mounts ( unsectioned tissues or cells) Freezing may be used instead of chemicals to fix tissues that need to be examined quickly or that have components damaged by the ch emicals Examples are frozen biopsies and tissues in which heat-labile structures are to be stained
- 24. Frozen specimens are sectioned using a cryot ome, a microtome encased in a freezing cha mber Permanent slides may be made from paraffin sections but not from frozen sections
- 25. Transmission Electron Microscopes TEM makes high-resolution ( ± 1 nm) views of the inner si de of objects. Mostly TEM is applied on material (e.g. cell s) that has been previously ' stained' and cut into ultrathi n sections, but sometimes al so intact objects < 1 µm, like viruses and aggregates of m acromolecules, are visualize d.
- 26. Principle Fixed, dehydrated specimens are embedded in a resin, hardened, sectioned, stained with heavy metals such as uranium and lead, a nd inserted into the electron column in the microscope The electron beam is absorbed or deflected by the heavy metal st ains and shadows are cast onto film or a phosphorescent plate at the bottom of the column - 2-D image - reveals internal cell structure - high resolution, high magnification - electron beam is focused by magnetic field
- 27. TEM images
- 28. Scanning Electron Microscope In SEM the image primary el ectrons from the source bo mbard the sample according to a scanning pattern and ca use emission of secondary e lectrons. In SEM an image of the surface of the object is made.
- 29. Principle Fixed, dehydrated specimens are mounted on stubs and surface-coated with gold, palladium or rhodium The specimen is placed in a vacuum and an electron beam scans back and fo rth over it Electrons that bounce off the metal-coated specimen surface are collected, c onverted to a digital image and displayed on a TV-like monitor - Electron beam is focused using a magnetic field - SEM provides a 3-D ima ge -Gives information about external topography of specimen -Much higher resolution and magnification than possible in light microscope
- 31. Micrograph A. Example of application of Transmission Electron Microscopy (TEM) Organelles in a pollen grain of tobacco (Nicotiana tabacum; AF = Actin filaments; G = Golgi apparatus; Mi = Mitochondrion; Mt = Microtubule). B. Example of application of Scanning Electron Microscopy (SEM) Overview of gills of a fish, the mudskipper (Periophthalmus argentilineatus).
- 32. Specimen Preparation Light Microscopy Live Mounts To view organisms, tissues, or cells in as close to the natural st ate as possible, unstained live Viewing time of live mounts is limi ted Unstained specimens have low contrast Supravital stains may be applied to provide more contrast or id entify certain components--these are stains that are not harmful to living cells mounts are used
- 33. Fixed (Preserved) Specimens for Hist ology--the Study of Tissue 1) Specimens may be preserved using chemicals such as f ormalin, acetic acid, ethanol, and methanol Fixation immobilizes molecules such as proteins and lipid s 2) Fixed specimens are dehydrated by serial transfer throu gh an ascending alcohol series, to 100% alcohol 3) Specimens are infiltrated with melted paraffin, paraffin substitute, or plastic and placed in a mold to harden
- 34. 4) Specimens are cut into 5-10 um thick sections usi ng a steel knife or razor on an instrument called a mi crotome 5) Sections are then mounted on slides, 6) Stained to achieve contrast or identify cell structur es or components, and 7) Viewed microscopically
- 36. Preparing Specimens for Scanning Electron Microscopy (SEM) 1) Fixation--fixatives used are glutaraldehyde, paraformaldehy de, osmium tetroxide 2) Dehydration is accomplished by carrying the specimens thro ugh an ascending alcohol series, to 100% alcohol (i.e., no water ), then to an organic solvent such as acetone or propylene oxide Specimens for SEM may also be processed in a critical point dr ying apparatus 3) Specimens are mounted on aluminum stubs using sticky tape
- 37. 4) A sputter coater coats the specimen with gold, pal ladium or rhodium in a special chamber to cover the sp ecimen with a 10-20 nm thick metal layer
- 38. 5) The stub is inserted into the SEM, scanned and obser ved on a video display
- 39. Preparing Specimens for Transmission El ectron Microscopy (TEM) 1) Fixation--specimens are fixed in glutaraldehyde, o r paraformaldehyde-glutaraldehyde mixtures, followe d by osmium tetroxide 2) Dehydration is accomplished by carrying the speci mens through an ascending alcohol series, to 100% alcohol (i.e., no water), then to an organic solvent su ch as acetone or propylene oxide 3) Specimens are then infiltrated with an epoxy or pl astic resin and placed in plastic molds to harden
- 41. 4) An instrument called an ultramicrotome is used to section the specimen
- 42. Glass or diamond knives are used to cut the ultrathi n sections
- 43. 5) Sections are transferred to tiny metal grids for support (the equivalent of th e function of the glass slide in LM) 6) Heavy metal stains such as uranyl acetate and lead citra te are applied to make certain structures electron dense
- 44. 7) Grids are then inserted into the transmission electron m icroscope and observed