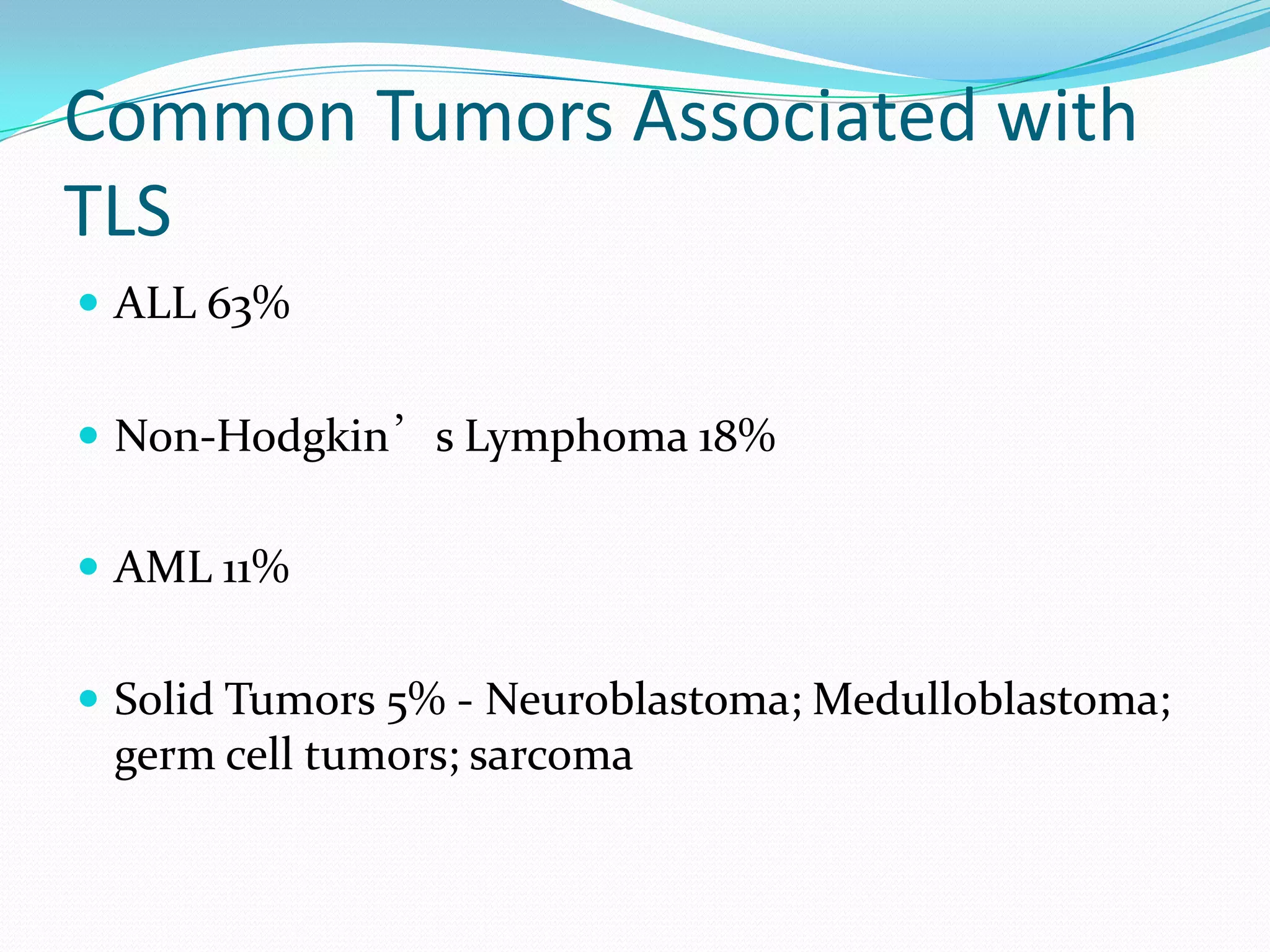
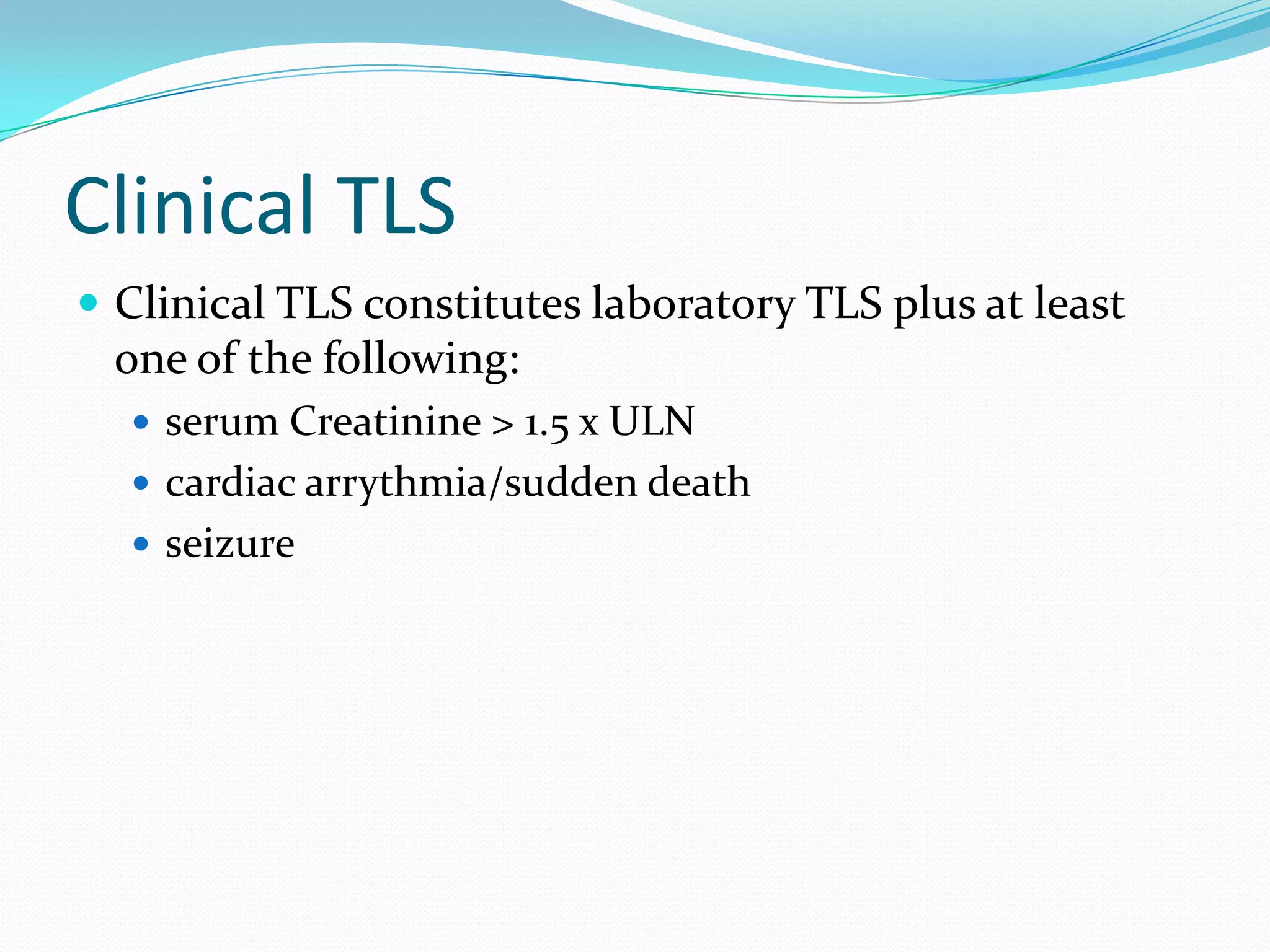
This document discusses tumor lysis syndrome (TLS), which can occur when tumors undergo rapid cell lysis and release intracellular contents into the bloodstream. TLS can cause electrolyte abnormalities and renal failure. It affects patients with highly proliferative tumors undergoing chemotherapy, radiation or other treatments. The document outlines risk factors, grading criteria, clinical manifestations, prevention strategies including hydration, uric acid reduction and dialysis, as well as treatment of established TLS complications. It also covers hyperleukocytosis, a related condition seen in some leukemia patients.