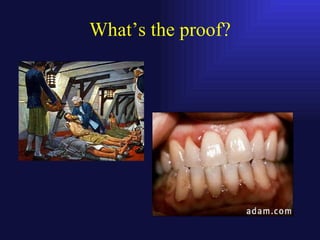
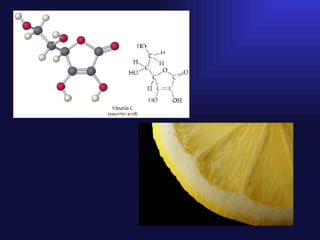
This document discusses collagen biochemistry. It covers the distribution of collagen in nature, the key collagen types, and collagen biosynthesis. Collagen biosynthesis involves post-translational modifications like lysine and proline hydroxylation. Collagen undergoes intracellular and extracellular processing, including removal of propeptide extensions and cross-linking by lysyl oxidase to form mature collagen fibers essential for tissue structure and integrity. Disorders like Ehlers Danlos syndrome result from defects in these collagen processing steps.