Embed presentation
Downloaded 294 times




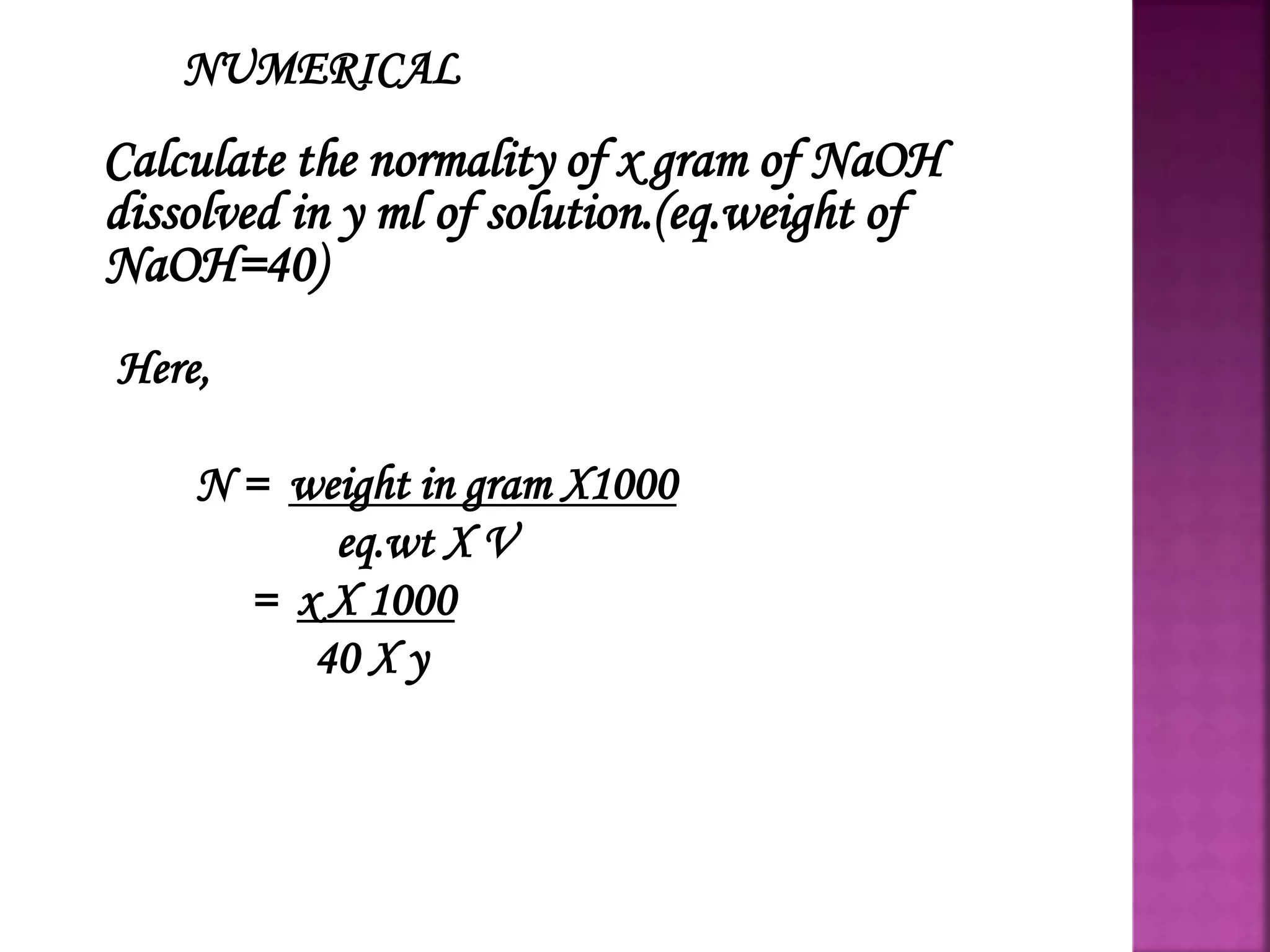



This presentation discusses normality, which is a measure of concentration defined as the number of gram equivalents of solute per liter of solution. A normal solution contains 1 gram equivalent of solute per liter. A decinormal solution contains 1/10 gram equivalent per liter. Normality depends on the amount of solute and temperature. It can be calculated using the formula: Normality = grams of solute x 1000 / (equivalent weight x volume of solution). The normality formula states that the normality of one solution multiplied by its volume equals the normality of a second solution multiplied by its volume. This formula can be used to calculate unknown normalities.







