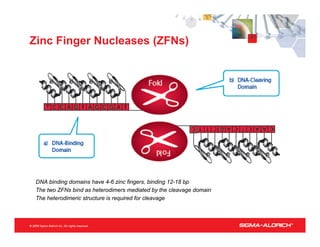
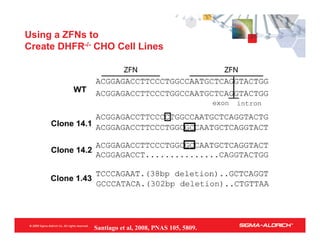
CompoZr™ ZFN technology allows rapid and precise engineering of mammalian cells for cGMP manufacturing of protein pharmaceuticals, featuring one-step gene knockout and targeted integration without selection. The technology is validated across multiple applications, improving efficacy and reducing costs in bioproduction, with commercial uses in therapeutic genome editing and enhancement of agricultural crops. ZFN technology enables bi-allelic modifications and offers significant advantages over traditional methods, including increased yield and reduced clonal variation.