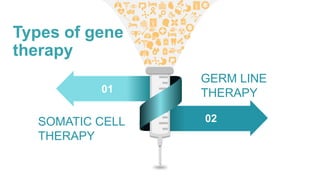
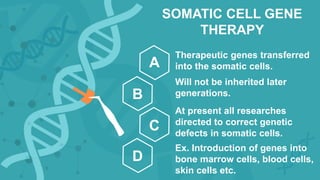
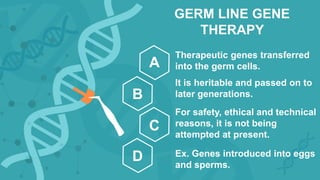
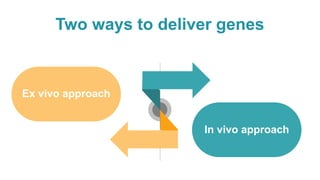
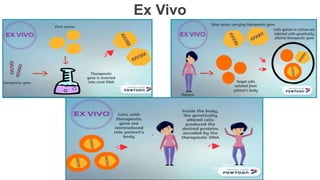
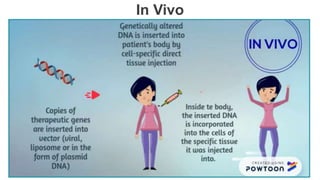
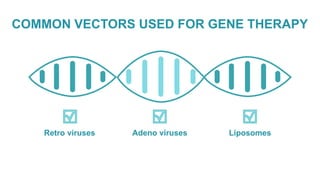
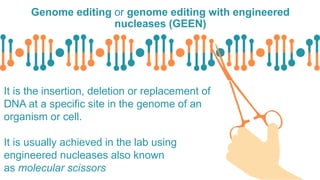
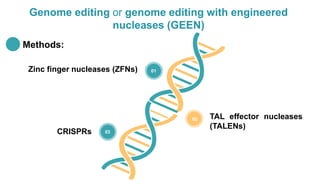
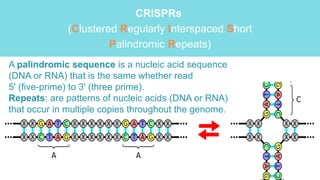
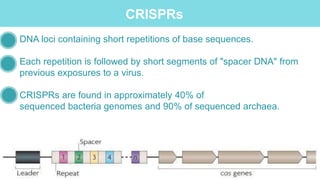
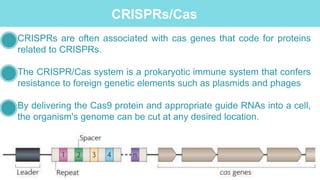
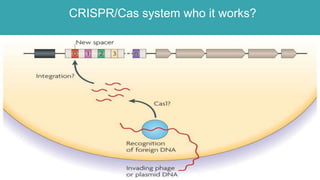
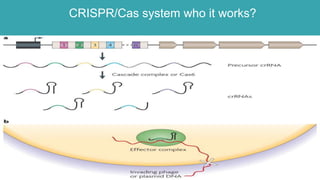
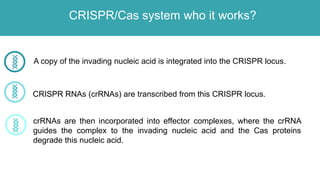
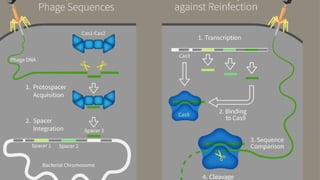
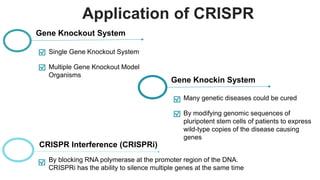
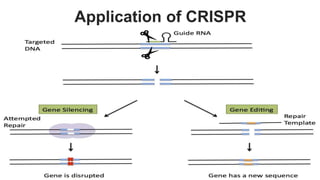
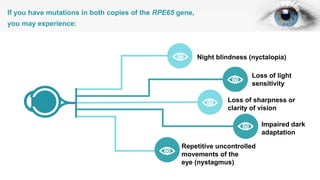
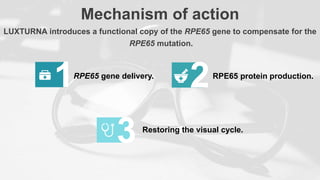
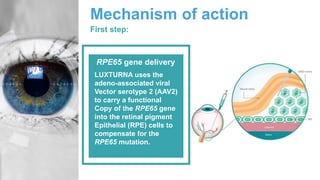
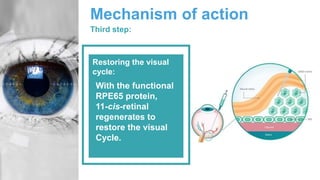
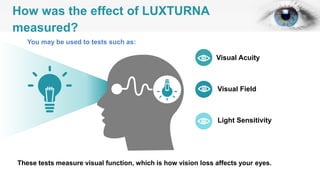
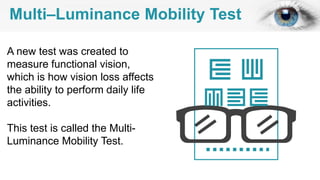
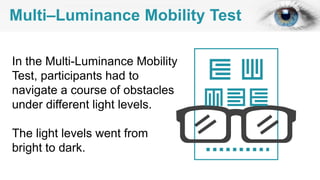
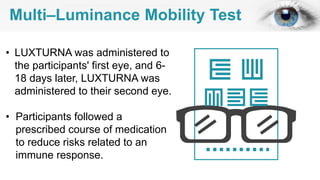
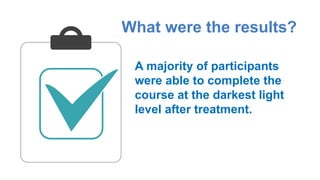
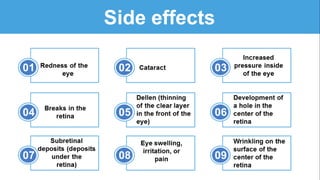
Gene therapy involves introducing genes into cells to treat disease. CRISPR is a gene editing technique using Cas9 protein and guide RNA. It allows DNA cutting and modification. Luxturna is a gene therapy for an inherited eye disease caused by RPE65 gene mutations. It uses AAV vector to insert a healthy RPE65 gene into retinal cells. In clinical trials, Luxturna improved patients' visual function and mobility in low light after 1 year as measured by a mobility test.